Structures and application of bifunctional protein and derivatives of bifunctional protein
A kind of use, protein technology, applied in the field of bioengineering, can solve the problems of linker length, immunogenicity toxicity, clinical treatment midway stop, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Molecular construction and sample preparation of recombinant proteins
[0067] The amino acid sequence of the recombinant protein is:
[0068] His-Gly-Glu-Gly-Thr-Phe-Thr-Ser-Asp-Val-Ser-Ser-Tyr-Leu-Glu-Gly-Gln-Ala-Ala-Lys-Glu-Phe-Ile-Ala-Trp- Leu-Val-Lys-Gly-Arg-Ala-Met-Gly-Ala-Tyr-Val-Tyr-Arg-Ser-Ala-Phe-Ser-Val-Gly-Leu-Glu-Thr-Tyr-Val-Thr- Ile-Pro-Asn-Met-Pro-Ile-Arg-Phe-Thr-Lys-Ile-Phe-Tyr-Asn-Gln-Gln-Asn-His-Tyr-Asp-Gly-Ser-Thr-Gly-Lys- Phe-His-Cys-Asn-Ile-Pro-Gly-Leu-Tyr-Tyr-Phe-Ala-Tyr-His-Ile-Thr-Val-Tyr-Met-Lys-Asp-Val-Lys-Val-Ser- Leu-Phe-Lys-Lys-Asp-Lys-Ala-Met-Leu-Phe-Thr-Tyr-Asp-Gln-Tyr-Gln-Glu-Asn-Asn-Val-Asp-Gln-Ala-Ser-Gly- Ser-Val-Leu-Leu-His-Leu-Glu-Val-Gly-Asp-Gln-Val-Trp-Leu-Gln-Val-Tyr-Gly-Glu-Gly-Glu-Arg-Asn-Gly-Leu- Tyr-Ala-Asp-Asn-Asp-Asn-Asp-Ser-Thr-Phe-Thr-Gly-Phe-Leu-Leu-Tyr-His-Asp-Thr-Asn
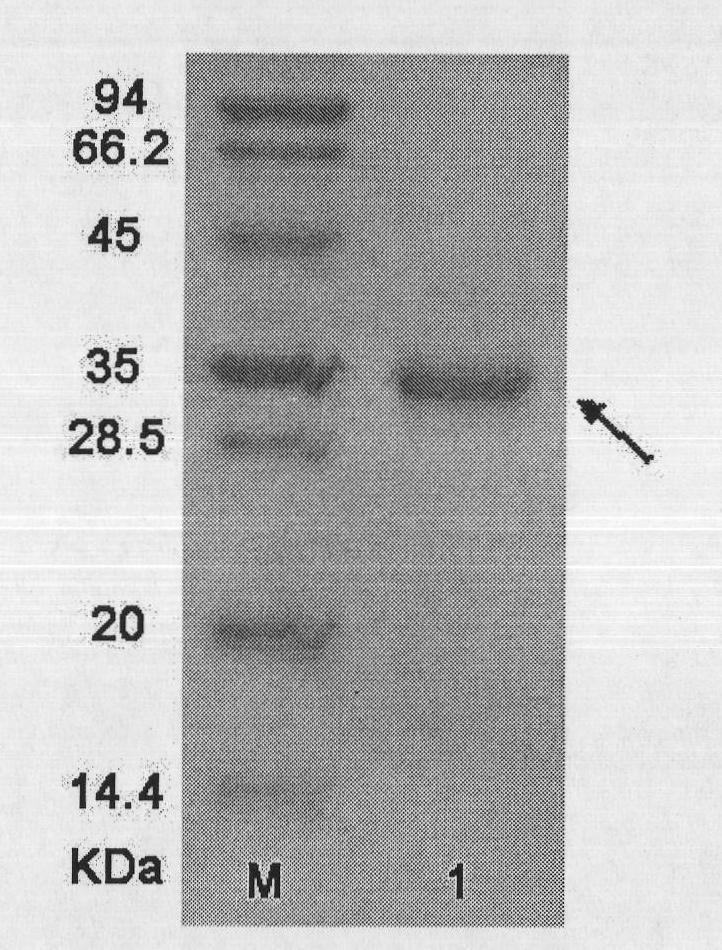
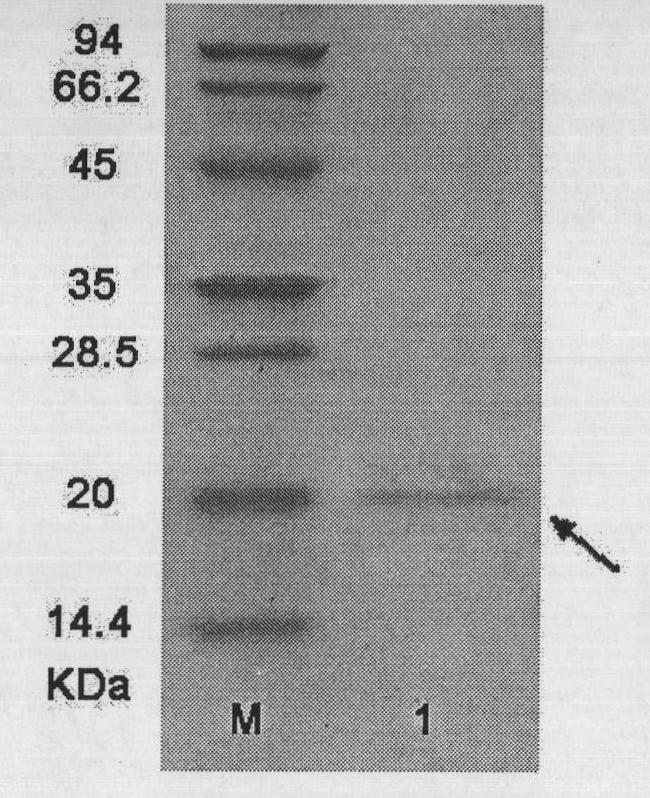
[0069] The recombinant protein has a total composition of 170 amino acids and a theoretical molecular weight of 19430.6 Daltons. According to the c...
Embodiment 2
[0078] Construction and preparation of recombinant proteins
[0079] The amino acid sequence of the recombinant protein is:
[0080] His-Gly-Glu-Gly-Thr-Phe-Thr-Ser-Asp-Leu-Ser-Lys-Gln-Met-Glu-Glu-Glu-Ala-Val-Arg-Leu-Phe-Ile-Glu-Trp- Leu-Lys-Asn-Gly-Gly-Pro-Ser-Ser-Gly-Ala-Pro-Pro-Pro-Ser-Gly-Met-Gly-Ala-Tyr-Val-Tyr-Arg-Ser-Ala-Phe- Ser-Val-Gly-Leu-Glu-Thr-Tyr-Val-Thr-Ile-Pro-Asn-Met-Pro-Ile-Arg-Phe-Thr-Lys-Ile-Phe-Tyr-Asn-Gln-Gln- Asn-His-Tyr-Asp-Gly-Ser-Thr-Gly-Lys-Phe-His-Cys-Asn-Ile-Pro-Gly-Leu-Tyr-Tyr-Phe-Ala-Tyr-His-Ile-Thr- Val-Tyr-Met-Lys-Asp-Val-Lys-Val-Ser-Leu-Phe-Lys-Lys-Asp-Lys-Ala-Met-Leu-Phe-Thr-Tyr-Asp-Gln-Tyr-Gln- Glu-Asn-Asn-Val-Asp-Gln-Ala-Ser-Gly-Ser-Val-Leu-Leu-His-Leu-Glu-Val-Gly-Asp-Gln-Val-Trp-Leu-Gln-Val- Tyr-Gly-Glu-Gly-Glu-Arg-Asn-Gly-Leu-Tyr-Ala-Asp-Asn-Asp-Asn-Asp-Ser-Thr-Phe-Thr-Gly-Phe-Leu-Leu-Tyr- His-Asp-Thr-Asn
[0081] The recombinant protein consists of 179 amino acids. According to the codon table of Escherichia coli and combined with...
Embodiment 3
[0088] The polyethylene glycol modification of the recombinant protein obtained in embodiment 1 and the preparation of the modified product
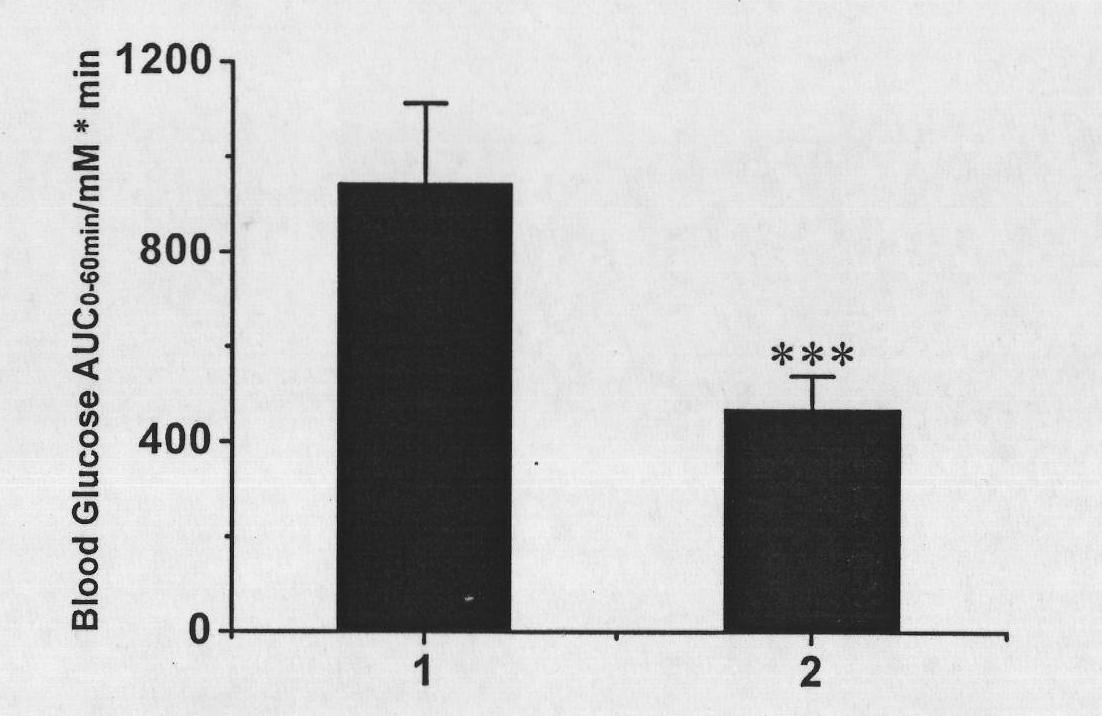
[0089] Accurately measure 10 mg (1 mg / ml, 10 ml) of the recombinant protein obtained in Example 1, and dialyze to replace the buffer system. After dialysis, the final reaction system was BufferR5 (100mMNaCl, 5mM EDTA, 20mMNa 2 HPO 4 -NaH 2 PO 4 , pH8.0). Add maleimide-activated polyethylene glycol (molecular weight 20,000 Daltons) to the reaction system at a molar ratio of 1:10, place the reaction mixture in a 4-degree thermostat for stirring reaction, and the rotating speed of the stirring device is set at 60 rpm. After 24 hours of reaction, the reaction mixture was diluted 1:5 (V / V) to Buffer D5 (5 mM EDTA, 20 mM Tris-HCl, pH 8.0) and purified by liquid chromatography.
[0090] The liquid phase system adopts GE Healthcare's AKTA medium and low pressure device, the chromatographic column is a 20×1.6 (I.D., cm) silanized glass colu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Theoretical molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com