Method for preparing thrombolytic medicament Reteplase without inclusion-body renaturation in escherichia coli
A technology of Escherichia coli and reteplase, which is applied in the field of biomedicine, can solve the problems of thrombolytic activity verification, affecting biological activity, difficulties, etc., and achieve the effect of simple and easy preparation method, efficient soluble expression, and reduced production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Construction of rPA Genetic Engineering Bacteria
[0030] 1. Using the pMD18T-tPA plasmid constructed in the previous research of our laboratory as a template (Jiang Jie, Jiang Chengying, Du Lianxiang, etc. Cloning of Reteplase gene and expression in Pichia methanolica. Journal of South China University of Technology, Natural Science Edition, 2006, 34(12):25-29).
[0031] Using upstream primers:
[0032] 5'-CGGg wxya ATCGAAGGTCGTTCTTACCAAGGAAACAGTG-3' (Sequence Listing; 1);
[0033] Downstream primers:
[0034] 5'-GGGctcgagATTACGGTCGCATGTTGTCACGAATCCAG-3', (Sequence Listing: 2) was amplified by PCR, and the amplified PCR product was detected by agarose gel electrophoresis, and purified and recovered by gel purification to obtain rPA gene fragments.
[0035]For the convenience of subsequent operations, BamHI and XhoI restriction sites (lowercase in bold) were introduced into the upstream and downstream primers respectively, and at the same time, factor Xa protease cu...
Embodiment 2
[0073] Induced expression of rPA in Escherichia coli
[0074] 1. Inoculate rPA recombinant Escherichia coli and Escherichia coli transformed with pET40b empty plasmid into 5 mL LB liquid medium containing kanamycin at a final concentration of 50 μg / mL, and culture overnight at 37°C and 220 rpm.
[0075] 2. The overnight culture obtained in the previous step was transferred to 200 mL of LB liquid medium containing kanamycin with a final concentration of 50 μg / mL at a volume ratio of 1%, and cultivated until its OD600 was 0.6 when adding IPTG ( isopropyl-β-D-thiogalactoside) or lactose for inducible expression. Specific optimal conditions for inducing expression: IPTG was induced at a final concentration of 0.6 mM at 25° C. for 3 h, and lactose was induced at a final concentration of 30 mM at 30° C. for 5 h.
[0076] 3. After the induction of expression, the bacterial liquid was collected separately, ultrasonically broken and centrifuged at 12000rpm, and the supernatant and pre...
Embodiment 3
[0079] Isolation and Purification of DsbC-rPA Fusion Protein
[0080] 1. Using the His-Tag on the pET-40b carrier, the fusion protein can be purified by Ni ion affinity chromatography. Firstly, nickel Sepharose FF was packed into a column, 1.6×20cm, with a column bed volume of 10mL.
[0081] 2. After equilibrating 2-5 bed volumes with buffer solution 1 (20mmol / L Tris-HCl pH7.9, 0.5mol / L NaCl, 10% glycerol) at 2mL / min, mix 20ml of cell disruption solution (50mM PBS, pH7 .4, 0.5M NaCl) 0.45μm membrane filtration, sample loading, the flow rate is 1mL / min.
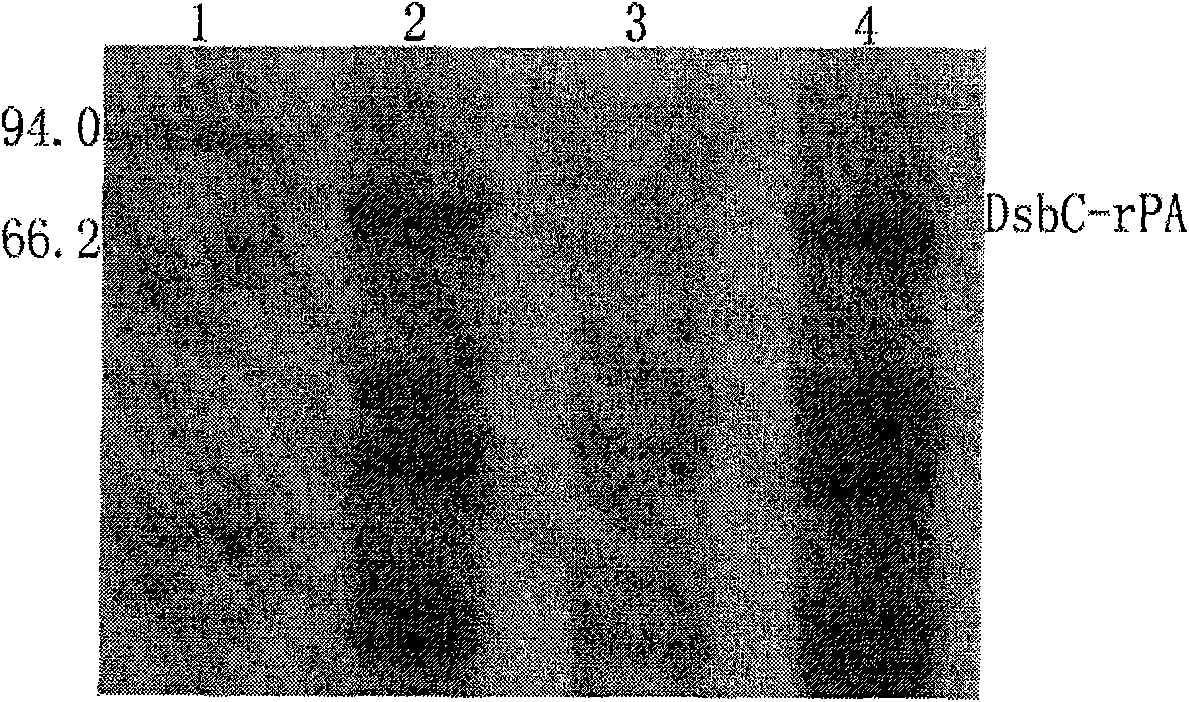
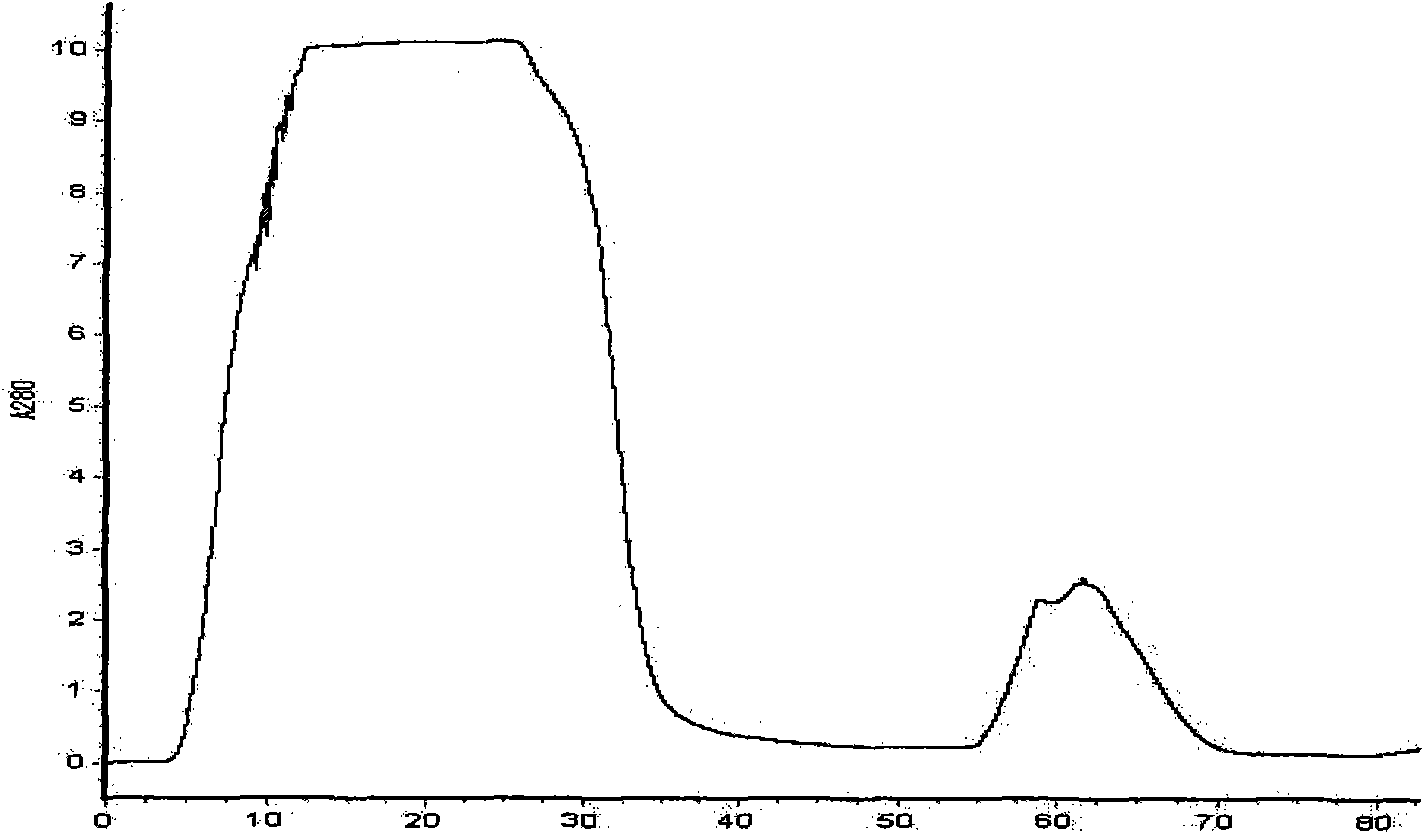
[0082] 3. Wash for another 2-5 bed volumes with Buffer 1 at a flow rate of 2 mL / min. Then use buffer 3 containing 10, 20, 50, 100, 200, 300, 400mM imidazole to carry out stage elution, the flow rate is 2mL / min, collect the elution peaks of each stage, and use SDS-PAGE to detect the molecular weight of the fusion protein and purity. The results showed that the fusion protein DsbC-rPA was mainly eluted from the nickel column...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com