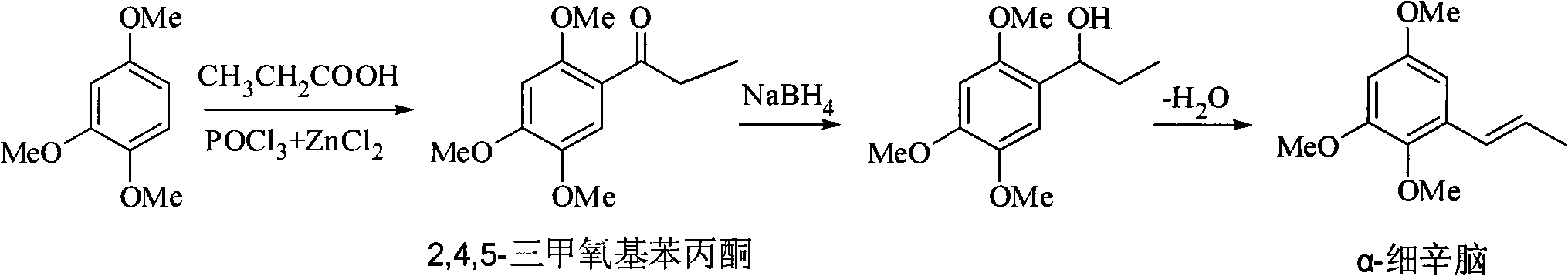
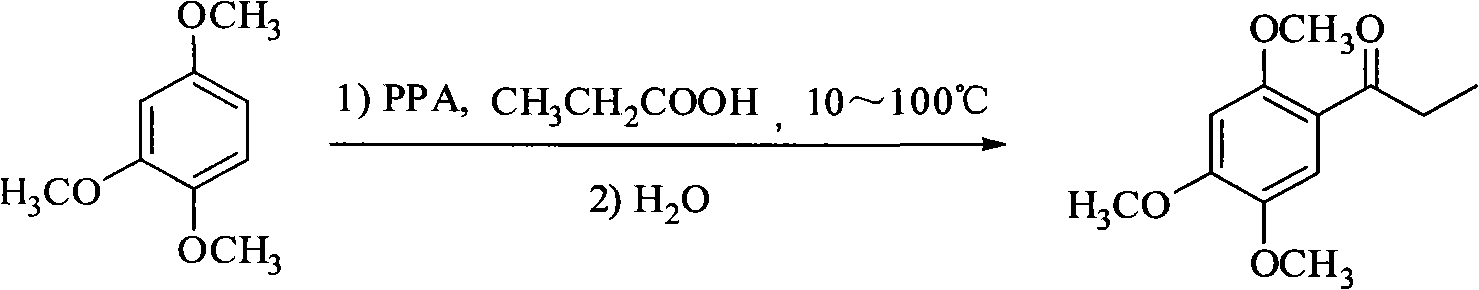Method for synthesizing 2,4,5-trimethoxyethylphenylketone intermediate of alpha-asarin
A technology of trimethoxybenzene and intermediates, which is applied in the field of the key intermediate 2 of the synthetic drug a-asarone, which can solve the problems of toxic organic solvents and cumbersome acetone operations, and achieve low cost, good product quality and safe operation reliable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Add 33.6 grams of 1,2,4-trimethoxybenzene and 25 grams of propionic acid, 400 grams of polyphosphoric acid (PPA) in a dry reactor, and stir the reaction at 45 ± 5 ° C, and detect the end point of the reaction with TLC (expansion system : n-hexane / ethyl acetate=8 / 2, concentrated sulfuric acid for color development), it takes about 5 hours to react, after the reaction is completed, cool to room temperature, pour the reactant into ice water, stir, and a large amount of solids are precipitated. For the amount of ice water, the mixed volume of ice and water is about 15-20 ml for every 10 grams of PPA used, or the mixed volume of ice and water is about 15-20 ml for every 10 grams of solvent used. Filter, wash with water until neutral, recrystallize with methanol, and dry to obtain 40.5 g of off-white solid, yield 90.4% (based on 1,2,4-trimethoxybenzene), mp 106-108°C.
Embodiment 2
[0037] Add 19.5 milliliters of 85% phosphoric acid and 30.6 grams of phosphoric anhydride into the dry reaction bottle and heat on a water bath until the phosphoric anhydride is completely dissolved, about 2 to 3 hours. Cool to room temperature, then add 1.4 grams of propionic acid, 1.68 grams of 1,2,4-trimethoxybenzene and 0.2 grams of sodium polyphosphate as an auxiliary agent, stir the reaction at 55±5°C, use TLC to detect the end of the reaction, and react for about 4 hours After the reaction is completed, cool to room temperature, pour the reactant into ice water, stir, and a large amount of solid precipitates out. Filter, wash with water until neutral, recrystallize with methanol, and dry to obtain 1.9 g of off-white product. Yield 84.8%, mp 106-108°C.
Embodiment 3
[0039] Add 5.04 grams of 1,2,4-trimethoxybenzene, 3 grams of propionic acid, 70 grams of polyphosphoric acid and 0.4 grams of auxiliary potassium dihydrogen phosphate into the dry reaction flask, and stir the reaction at 85±5°C. The end point of the reaction was detected by TLC. After the reaction was completed for about 3 hours, the reaction was cooled to room temperature, and the reactant was poured into ice water, stirred, and a large amount of solids were precipitated. Filter, wash with water until neutral, recrystallize with methanol, and dry to obtain 6 g of off-white solid product with a yield of 89.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com