Agkistrodon halys venom thrombin and preparation method and application thereof
A technology of thrombin and snake venom, which is applied in the field of snake venom thrombin and its preparation, can solve the problems of softness and instability, and achieve high stability and safety, high safety, and good hemostatic performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] (1) DEAE-Sepharose Fast Flow chromatography
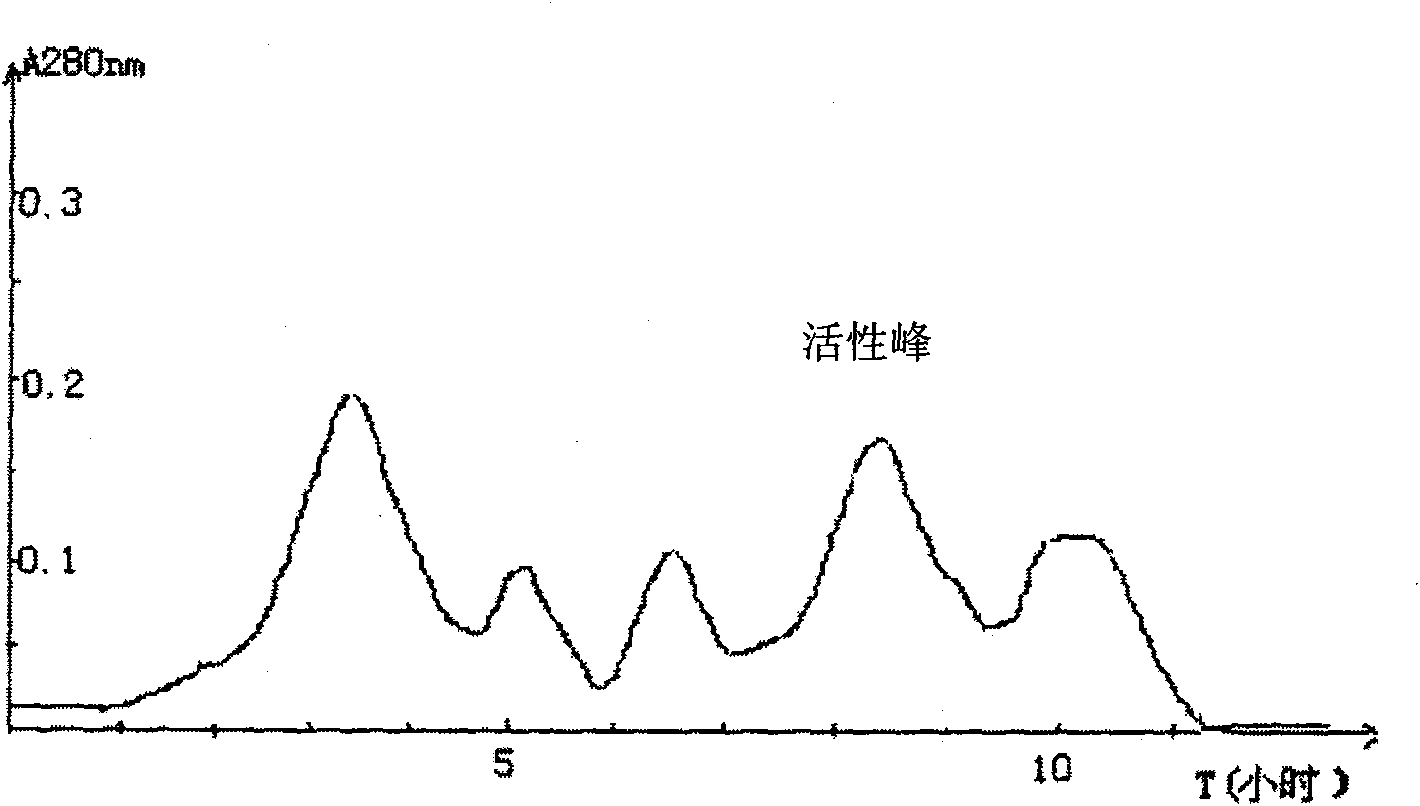
[0055] Weighed 20 grams of snake venom purchased from Agkistrodon blomhoffii brevicaudus in Liaoning, dissolved it in 0.05 mol / L pH 8.0 Tris-HCl buffer, and centrifuged at 6000 rpm / min for 15 minutes to obtain a supernatant. Put the supernatant on a well-balanced DEAE-Sepharose Fast Flow chromatography column, first use 0.05mol / L Tris-HCl buffer to elute unadsorbed impurities, and then use a buffer solution containing 0-0.6mol / L NaCl for gradient Elution, collect each peak solution, take 0.1ml of each solution and add bovine plasma diluted twice with normal saline, if the plasma coagulates, it is the peak of thrombin activity. See figure 2 .
[0056] (2) Affinity chromatography
[0057] Put the active component collection solution of the above-mentioned thrombin activity peak on a well-balanced affinity chromatography column, first elute unadsorbed impurities with 0.05mol / L Tris-HCl pH8.0 buffer containing 0.5mol / L NaCl;...
Embodiment 2
[0064] The measurement result of the Agkistrodon halys venom thrombin made by embodiment 1 is as follows:
[0065] 1) Measured by SDS-polyacrylamide gel electrophoresis, a single band can be seen in the electropherogram, and the molecular weight is 32.3Kda. See Figure 5 .
[0066] 2) Determination of chromatographic conditions and system suitability test by high performance liquid chromatography: gel chromatography column: TSK GEL2000SWxl7.8mmX300mm, 5μm; mobile phase: 0.2mol / L sodium phosphate buffer (pH6.8); detection wavelength: 280nm ;; Injection volume: 20 μL; Column temperature: room temperature; Flow rate: 1.0ml / min. As a result, the purity was 98.56%. See Image 6 .
[0067] 3) As determined by the Procise 491 sequencer of the American ABI company, the enzyme consists of 236 amino acids, and the amino acid sequence is shown in SEQ ID NO: 1 (see figure 1 ).
[0068] 4) Activity measurement: the amount of enzyme that can coagulate 1.0ml of oxalated bovine whole b...
Embodiment 3
[0071] The thrombin of the invention can coagulate human plasma and bovine fibrinogen in vitro, and the coagulation specific activity of plasma is greater than 600 units / mg. The clot was dispersed in 5M urea solution, indicating that Agkistrodon venom thrombin did not activate coagulation factor XIII, and produced cross-linked fibrin, which could be rapidly degraded and cleared by plasmin.
[0072] Effect of intraperitoneal injection of Agkistrodon venom thrombin on the bleeding time of mice: Mice were intraperitoneally injected with normal saline, Agkistrodon venom thrombin, and Helizhizhi. 30 minutes after the administration, the tail of the mouse was quickly cut at 0.5 cm from the tip of the tail, and the outflowing blood was sucked up with filter paper regularly. The bleeding time was from the beginning of cutting the tail to the end of no bleeding. The results are shown in Table 2.
[0073] Table 2 Effect of intraperitoneal injection of Agkistrodon venom thrombin on blee...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com