Method for producing granulated matter
A manufacturing method and technology of granule preparations, which are applied in the field of granule preparation manufacturing and can solve problems such as instability and loss of stability of unstable substances
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
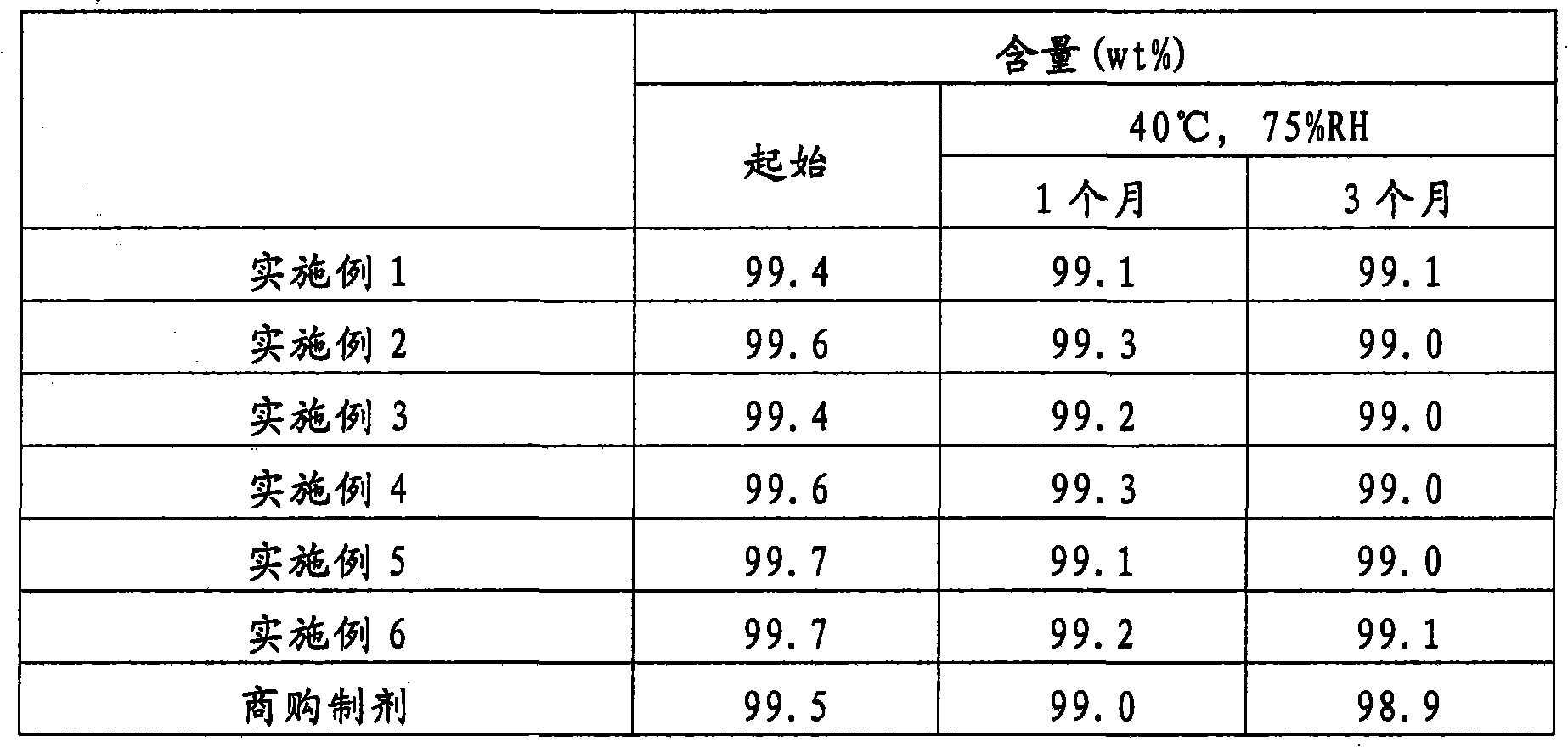
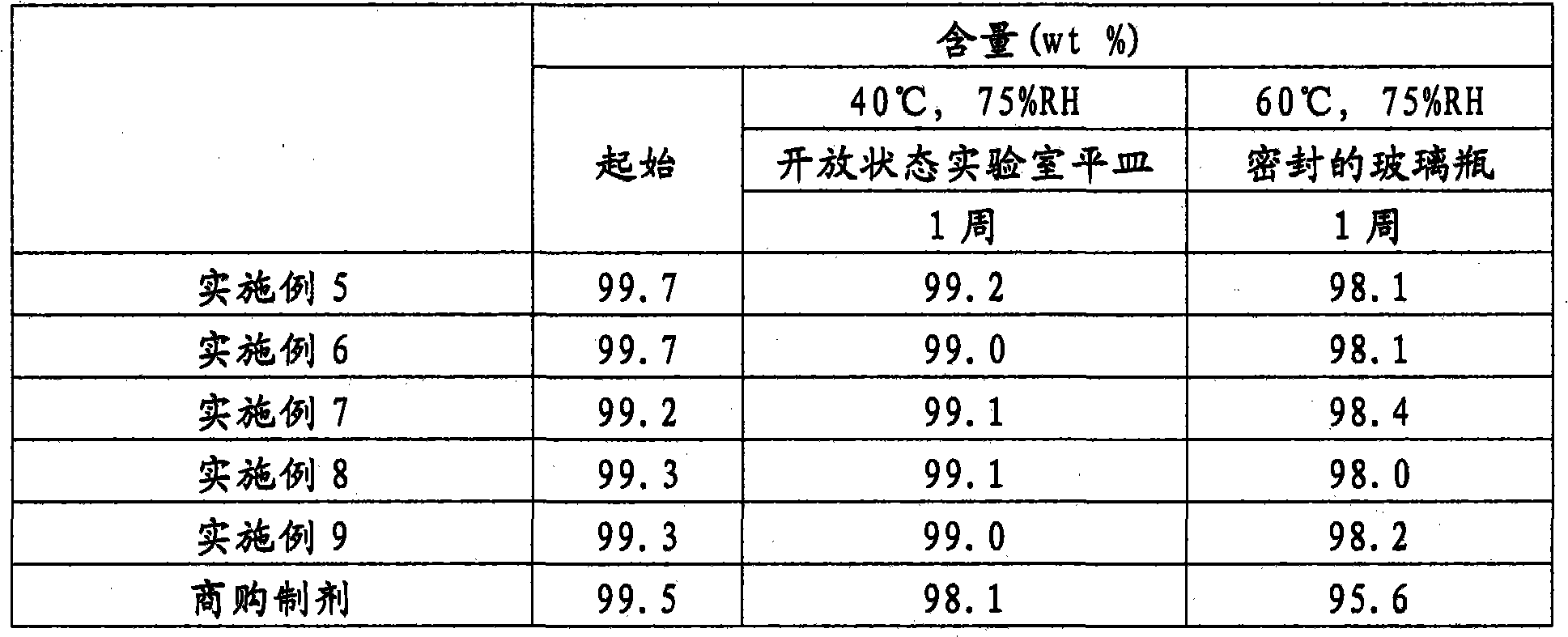
[0037] 356.0 g of magnesium oxide was charged into a jet fluidized bed granulator (Model MP-01-SPC, manufactured by Powrex Corporation), followed by fluidization. The granules were produced by spraying a liquid prepared by dissolving 80.0 g of rabeprazole sodium in a solution of 20.0 g of sodium hydroxide in 220.0 g of pure water on a fluidized bed, followed by drying. The granules were made into a uniform size (stable uniform granules) by passing through a JIS 24-mesh sieve. 114.0 g of the obtained stable uniform granules were fed into a jet fluidized bed granulator, and the granules were coated by spraying a liquid by dissolving and suspending 11.0 g of polyvinyl alcohol copolymer and 11.0 g of talc in 253.0 g of pure water was prepared and then dried. The granules were then coated by spraying a liquid prepared by dissolving and suspending 62.0 g of methacrylic acid copolymer LD (30% by weight liquid), 9.6 g of talc and 1.8 g of triethyl citrate in purified water , then dr...
Embodiment 2
[0049] Into the same jet fluidized bed granulator as used in Example 1, 406.0 g of D-mannitol was added, followed by fluidization. The particles were then produced by spraying a liquid prepared by dissolving 40.0 g of rabeprazole sodium having an average particle diameter of 5 μm in a solution of 10.0 g of sodium hydroxide dissolved in 110.0 g of pure water, followed by drying. The granules were made into a uniform size (stable uniform granules) by passing through a JIS 24-mesh sieve. Add 228.0 g of the obtained stable and uniform granules into a jet fluidized bed granulator, and coat the granules by spraying a liquid that dissolves and suspends 22.0 g of polyvinyl alcohol and 22.0 g of talc in 506.0 g of pure Water is prepared and then dried. The granules were then coated by using a liquid by dissolving and suspending 124.0 g of methacrylic acid copolymer LD (30 W / W% liquid), 19.2 g of talc and 3.6 g of triethyl citrate in purified water prepared, then dried. The obtained ...
Embodiment 3
[0061] To the same jet fluidized bed granulator as used in Example 1 was added 414.0 g of D-mannitol. Particles were then produced by using a liquid prepared by dissolving 40.0 g of rabeprazole sodium having an average particle diameter of 5 μm in a solution of 2.0 g of sodium hydroxide dissolved in 110.0 g of pure water, followed by drying. The granules were made into a uniform size (stable uniform granules) by passing through a JIS 24-mesh sieve. As a result of operating in the same manner as in Example 1, an enteric granule preparation having the composition given below was obtained.
[0062] [Ingredient] [Weight (mg) based on 500mg granule preparation]
[0063] Rabeprazole Sodium 10.0
[0064] D-mannitol 103.5
[0066] Polyvinyl alcohol copolymer 11.0
[0067] Talc 20.6
[0068] Methacrylic acid copolymer LD 18.6
[0069] Triethyl citrate 1.8
[0070] D-mannitol 314.0
[0071] Hydroxypropyl Cellulose 20.0
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com