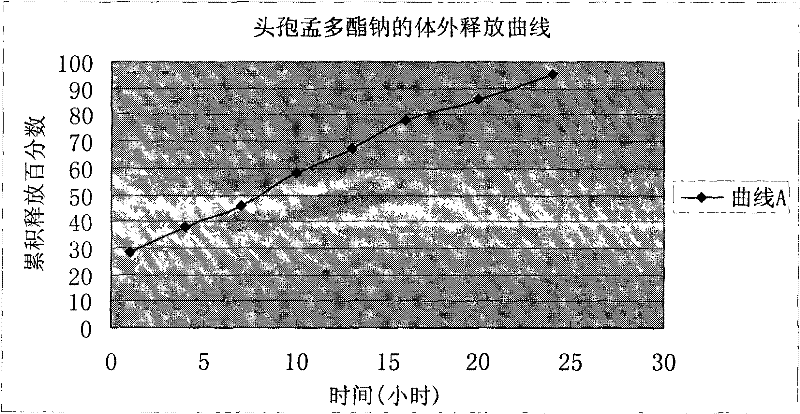
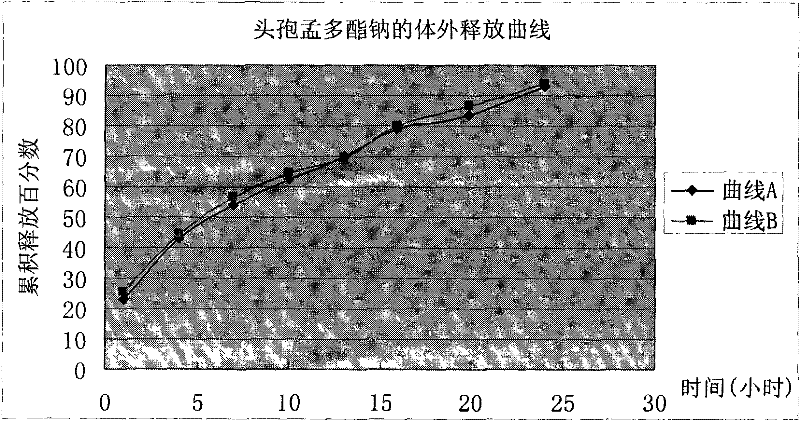
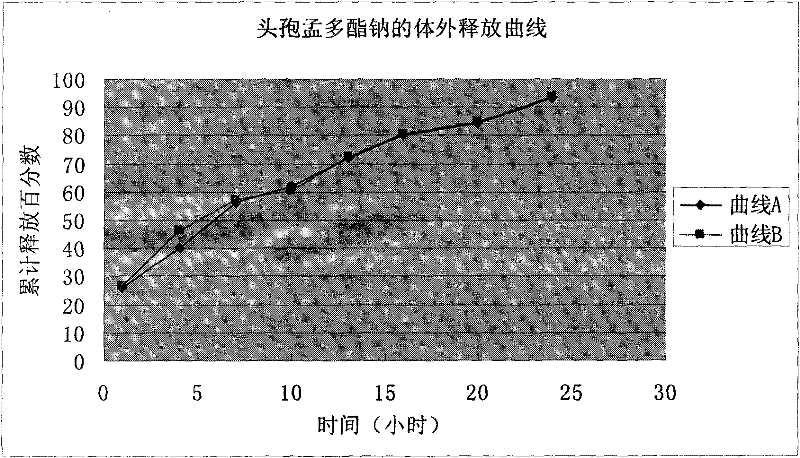
Cefamandole nafate/anhydrous sodium carbonate medicinal composition microsphere injection
A technology of cefamandole sodium and anhydrous sodium carbonate, which is applied in the field of medicine and can solve the problems of low yield of liposome and suspension preparation, unclear clinical effect, and complicated preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] The preparation of embodiment 1 cefamandole sodium / anhydrous sodium carbonate injection
[0061] Prescription: (100 bottles)
[0062] Cefamandole Sodium 50g
[0063] Anhydrous sodium carbonate 4g
[0064] PGLA 75g
[0065] Propylene glycol 15g
[0066] Tween 80 10g
[0067] Glucose 100g
[0068] Preparation Process:
[0069] (1) 50g cefamandole sodium, 15g propylene glycol and 100g glucose are dissolved in 500ml water for injection to obtain the aqueous phase;
[0070] (2) 75g PGLA and 10g Tween 80 were dissolved in 500ml of chloroform and isopropanol organic solvent with a volume ratio of 1:1 to obtain an oily phase;
[0071] (3) Slowly drop the water phase obtained above into the oil phase under stirring conditions, control the dropping speed to 5ml / min, stir for 10min after dropping, then transfer to a high-speed homogenizer and stir at a high speed for 3 times at a speed of 15000r / min, each 10min, to obtain a uniform white emulsion;
[0072] (4) Put the wh...
Embodiment 2
[0076] The preparation of embodiment 2 cefamandole sodium / anhydrous sodium carbonate injection
[0077] Prescription: (100 bottles)
[0078] Cefamandole Sodium 100g
[0079] Anhydrous sodium carbonate 12g
[0080] PGLA 600g
[0081] Propylene glycol 300g
[0082] Tween 80 200g
[0083] Glucose 800g
[0084] Preparation Process:
[0085] (1) 100g cefamandole sodium, 300g propylene glycol and 800g glucose are dissolved in 2000ml water for injection to obtain the aqueous phase;
[0086] (2) 600g PGLA and 200g Tween 80 are dissolved in 2000ml of chloroform and isopropanol organic solvent with a volume ratio of 1:1 to obtain an oily phase;
[0087] (3) Slowly drop the water phase obtained above into the oil phase under stirring conditions, control the dropping speed to be 10ml / min, stir for 30min after dropping, then transfer to a high-speed homogenizer for high-speed stirring for 5 times at a speed of 15000r / min, each 5min, to obtain a uniform white emulsion;
[0088] (4...
Embodiment 3
[0092] The preparation of embodiment 3 cefamandole sodium / anhydrous sodium carbonate injection
[0093] Prescription: (100 bottles)
[0094] Cefamandole Sodium 200g
[0095] Anhydrous sodium carbonate 20g
[0096] PGLA 800g
[0097]Propylene glycol 400g
[0098] Tween 80 300g
[0099] Glucose 1000g
[0100] Preparation Process:
[0101] (1) 200g cefamandole sodium, 400g propylene glycol and 1000g glucose are dissolved in 4000ml water for injection to obtain the aqueous phase;
[0102] (2) 800g PGLA and 400g Tween 80 were dissolved in 3000ml of chloroform and isopropanol organic solvent with a volume ratio of 1:1 to obtain an oily phase;
[0103] (3) Slowly drop the water phase obtained above into the oil phase under stirring conditions, control the dropping speed to 8ml / min, stir for 20min after dropping, then transfer to a high-speed homogenizer for high-speed stirring 4 times at a speed of 15000r / min, 7min each time, to obtain a uniform white emulsion;
[0104] (4)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com