Application of organic amine derivatives as brain-targeting modification group of small-molecule drug
An organic amine and brain-targeting technology, applied in the field of medicine, can solve the problems of low permeability, limited dosage, complicated preparation process, and many blood-brain barrier complications, and achieves easy mass production, simple connection method, good The effect of applying the foreground
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
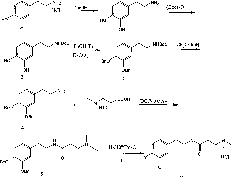
[0062] Ibuprofen is heated to reflux reaction at 60° C. in excess thionyl chloride solution until no hydrogen chloride gas overflows. After the reaction, excess thionyl chloride was removed by distillation under reduced pressure to obtain ibuprofenyl chloride, which was directly used in the following reaction.
Embodiment 2
[0064] Synthesis of 2-methylaminoethanol-ibuprofen ester (II)
[0065] Dissolve 0.5g (7.0mmol) of 2-(methylamino)ethanol and 0.28g (7.0mmol) of sodium hydroxide in 10.0ml of methanol, add 1.90g (8.6mmol) of BOC anhydride under stirring, and continue stirring at room temperature Reaction 5h, stop the reaction. Spin to dry under reduced pressure, the residue was separated by silica gel H flash column, dichloromethane:methanol (20:1) was eluted, and the collected solution was concentrated under reduced pressure to obtain 0.86 g of a colorless oily liquid, which was N-(methyl)-β - tert-butyl hydroxyethyl carbamate, yield 71.1%.
[0066] Dissolve 0.7g (4.1mmol) tert-butyl N-(methyl)-β-hydroxyethylcarbamate in 10ml of dichloromethane, slowly add 1.10g (4.9mmol) ibuprofenyl chloride dropwise under stirring, dropwise After completion, 0.50 g (4.9 mmol) of triethylamine was added dropwise, and stirring was continued at room temperature for 4.5 h to stop the reaction. Spin to dry und...
Embodiment 3
[0071] Synthesis of 2-dimethylaminoethanol ibuprofen ester (III)
[0072] Dissolve 1.30g (14.6mmol) of N,N-dimethylethanolamine and 5ml of triethylamine in 10ml of dichloromethane, and slowly add 2.24g (10mmol) of ibuprofenyl chloride dropwise under ice-bath conditions, and the addition is complete Afterwards, the reaction was continued at room temperature for 4 h, extracted three times with dichloromethane, and the dichloromethane solution was combined. The organic layer was washed with aqueous sodium bicarbonate and aqueous sodium chloride, dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure. Flash column separation, eluting with dichloromethane:methanol (20:1), concentrated the collected solution under reduced pressure to obtain 2.32 g (83.7%) of a colorless oily liquid
[0073] 1HNMR (400MHz, CDCl3): 7.178 (d, 2H, J=8.8Hz, 2'-H, 6'-H), 7.059 (d, 2H, J=8.0Hz, 3'-H, 5'-H) , 4.191-4.107(m, 2H, CH2CH2OCO), 3.660-3.720(m, 1H, COCHCH3), 2.503(...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com