Method for preparing potassium citrate sustained-release tablets
A technology of potassium citrate and sustained-release tablets, which is applied in the field of medicine and achieves the effects of excellent sustained-release characteristics, reduced administration times, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
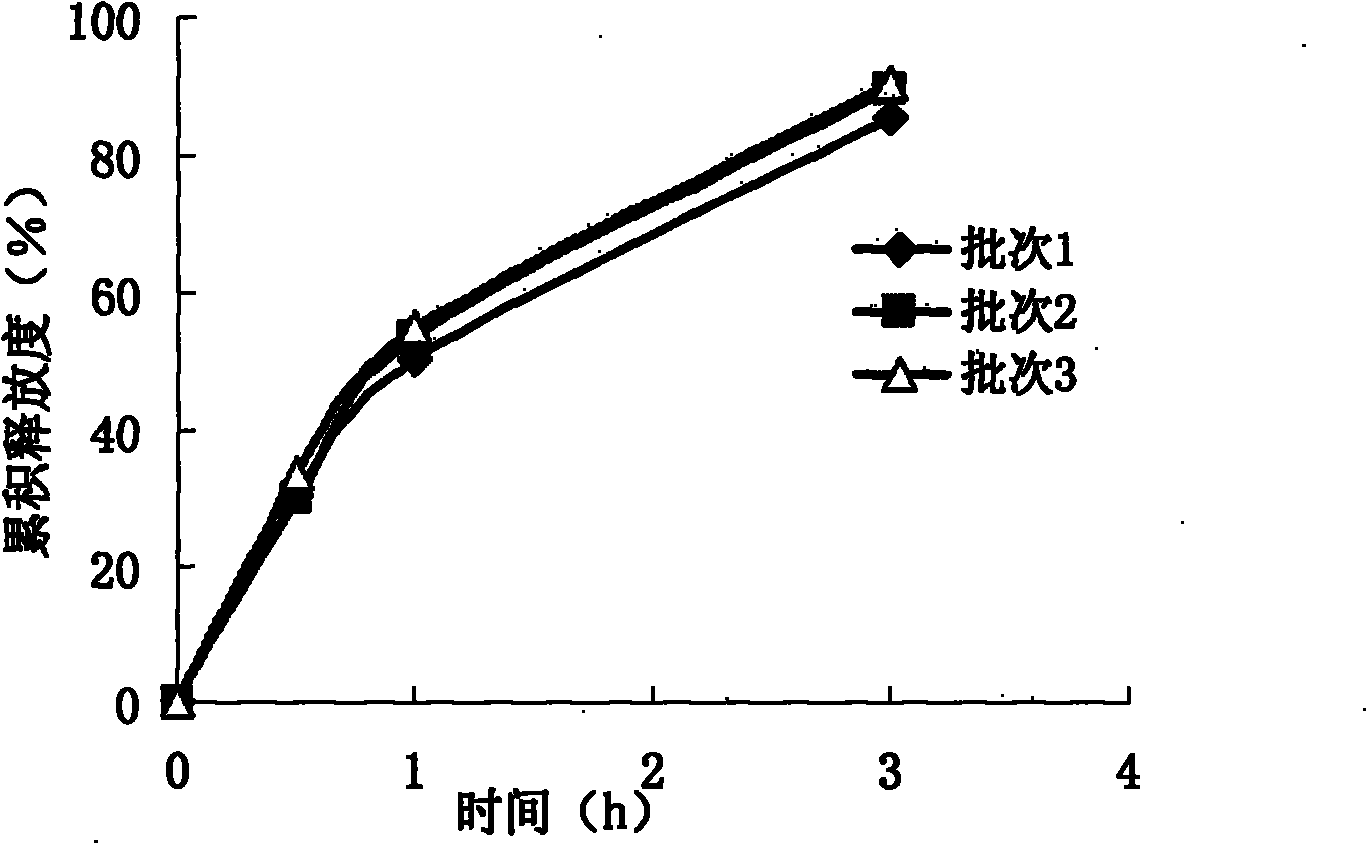
Image
Examples
Embodiment 1
[0032] Potassium citrate 57.1g
[0033] Ethyl cellulose 25.0g
[0034] Polyethylene glycol 6000 5.0g
[0035] Povidone K 30 Appropriate amount (0.01%-3% of the above total weight)
[0036] Pigment Appropriate amount (0.001%-1% of the above total weight)
[0037] The preparation process is as follows: Potassium citrate, ethyl cellulose and polyethylene glycol 6000 are weighed according to the prescription and passed through a 80-mesh sieve. After mixing evenly, add the prepared 5% povidone K 30 Appropriate amount of aqueous solution to prepare soft materials, granulate with 16 mesh sieve, dry at 50-60°C for 2 hours, granulate with 18 mesh sieve, mix well, punch tablets with 12mm shallow concave, control the hardness at about 60-80N, and make 100 tablets.
Embodiment 2
[0039] Potassium citrate 571g
[0040] Stearic acid 200g
[0041] Macrogol 4000 40g
[0043] Povidone K 30 Appropriate amount (0.01%-3% of the above total weight)
[0044] Pigment Appropriate amount (0.001%-1% of the above total weight)
[0045] The preparation process is as follows: Potassium citrate, stearic acid and polyethylene glycol 4000 are weighed according to the prescription and passed through an 80-mesh sieve. After mixing evenly, add the prepared 5% povidone K 30 Appropriate amount of aqueous solution, prepare soft material, granulate with 16 mesh sieve, dry at 50-60°C for 2 hours, granulate with 18 mesh sieve, add the prescribed amount of magnesium stearate, mix well, punch tablets with 12mm shallow concave, the hardness is controlled at 60~ About 80N, 100 pieces were prepared.
Embodiment 3
[0047] Potassium citrate 571g
[0048] Glyceryl monostearate 60g
[0049] Stearic acid 150g
[0050] Lactose 100g
[0052] Povidone K 30 Appropriate amount (0.01%-3% of the above total weight)
[0053] Pigment Appropriate amount (0.001%-1% of the above total weight)
[0054] The preparation process is: weigh potassium citrate, stearic acid, glyceryl monostearate and lactose according to the prescription, put them into a fluidized one-step granulator, increase the temperature to 50-60 degrees, and spray 5% Povidone K 30 Appropriate amount of aqueous solution, prepare granules, dry at 50-60°C, repeat this process, sieve the prepared granules with 16 mesh, add the prescribed amount of magnesium stearate, mix well, punch tablets with 12mm shallow flat concave, the hardness is controlled at 60 ~80N or so, make 800 pieces.
[0055] Certainly the method for this preparation also can realize by the method for embodiment 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com