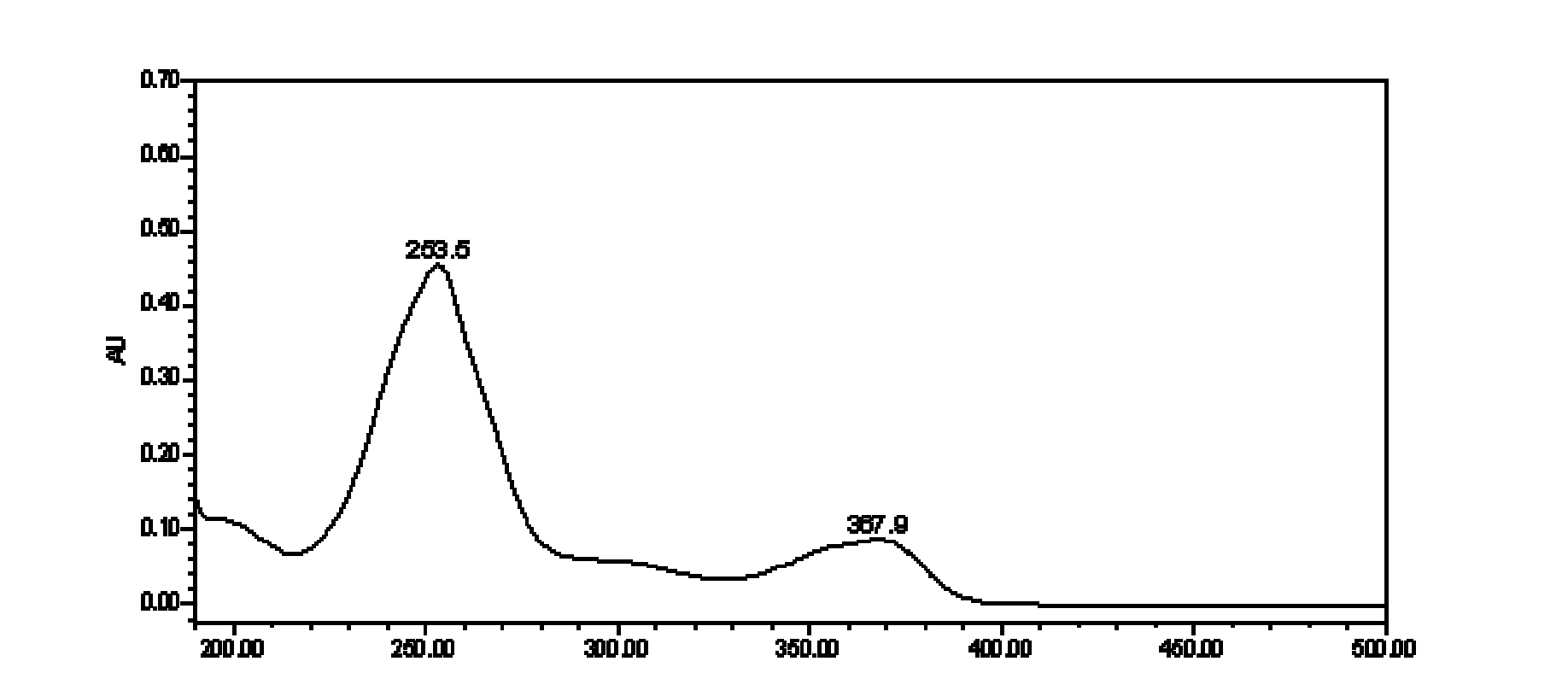
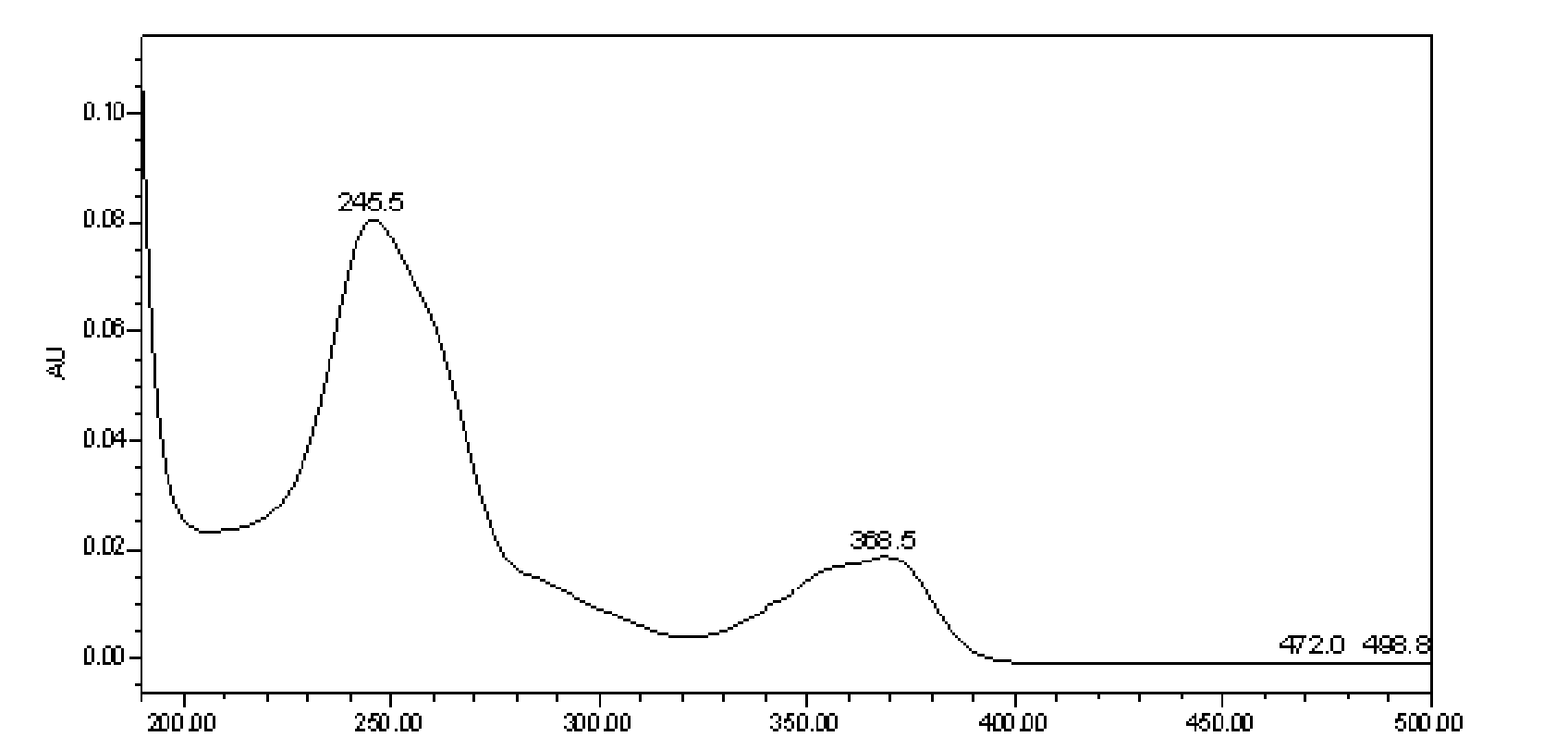
Method for measuring content of ellagic acid ingredients in euscaphis japonica medicinal materials
A determination method and technology of clavus, applied in the field of content determination of ellagic acid components in clavus medicinal materials, can solve problems such as less research on clavus medicinal materials, weak basic research, lack of qualitative and quantitative method reference substances, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0128] Embodiment 1 of the present invention: the content determination method of ellagic acid composition in clavus medicinal material is:
[0129] Adopt ultra-high performance liquid chromatography, specifically comprise the following steps:
[0130] (1) Chromatographic conditions and system suitability test:
[0131] Instrument: Waters ACQUITY UPLC chromatograph (including binary gradient pump, vacuum degasser, autosampler, column oven, diode array detector, Epower 2 workstation);
[0132] Chromatographic column: ACQUITY UPLC BEH C18 column (2.1mm×100mm, 1.7μm);
[0133] Mobile phase: A is acetonitrile, B is 0.1% H 3 PO4, carry out gradient elution according to A:B=6~100:94~0; during the gradient elution process of the mobile phase, the phase A and phase B of the eluent change as follows: at the beginning, phase A is 6%, phase B is 94% , stable for 1 minute; at the 19th minute, phase A was 35%, and phase B was 65%; at the 22nd minute, phase A was 100%, and phase B was 0;...
Embodiment 2
[0144] Embodiment 2 of the present invention: the method for determining the content of ellagic acid components in clavus medicinal materials can also be:
[0145] Adopt ultra-high performance liquid chromatography, specifically comprise the following steps:
[0146] (1) Chromatographic conditions and system suitability test:
[0147] Instrument: Waters ACQUITY UPLC chromatograph;
[0148] Chromatographic column: ACQUITY UPLC BEH C18 column;
[0149] Mobile phase: A is acetonitrile, B is 1% H 3 PO4, carry out gradient elution according to A:B=6~100:94~0; during the gradient elution process of the mobile phase, the phase A and phase B of the eluent change as follows: at the beginning, phase A is 6%, phase B is 94% , stable for 1 minute; at 19 minutes, phase A is 35%, and phase B is 65%; at 22 minutes, phase A is 100%, and phase B is 0.
[0150] Flow rate: 0.2mL / min;
[0151] Column temperature: 35°C;
[0152] Detection wavelength: 235~250nm;
[0153] Injection volume: 1μ...
Embodiment 3
[0158] Embodiment 3 of the present invention: the method for determining the content of ellagic acid components in clavus medicinal materials can also be:
[0159] Adopt ultra-high performance liquid chromatography, specifically comprise the following steps:
[0160] (1) Chromatographic conditions and system suitability test:
[0161] Instrument: Waters ACQUITY UPLC chromatograph;
[0162] Chromatographic column: ACQUITY UPLC BEH C18 column;
[0163] Mobile phase: A is acetonitrile, B is 0.5% H3PO4, gradient elution is carried out according to A:B=6~100:94~0; during the gradient elution process of the mobile phase, the phase changes of A and B of the eluent are: start At 22 minutes, phase A is 100% and phase B is 0.
[0164] Flow rate: 0.2mL / min;
[0165] Column temperature: 45°C;
[0166] Detection wavelength: 240~260nm;
[0167] Injection volume: 1μL;
[0168] The number of theoretical plates is calculated to be above 160,000.
[0169] (2) Preparation of reference solu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com