Application of polymer based on beta-cyclodextrin
A polymer and cyclodextrin technology, which is applied in the directions of inactive medical preparations, inactive polymer compounds, and medical preparations containing active ingredients, etc. problems, to achieve good biocompatibility, inhibition of P-gp effect, and the effect of reversing drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
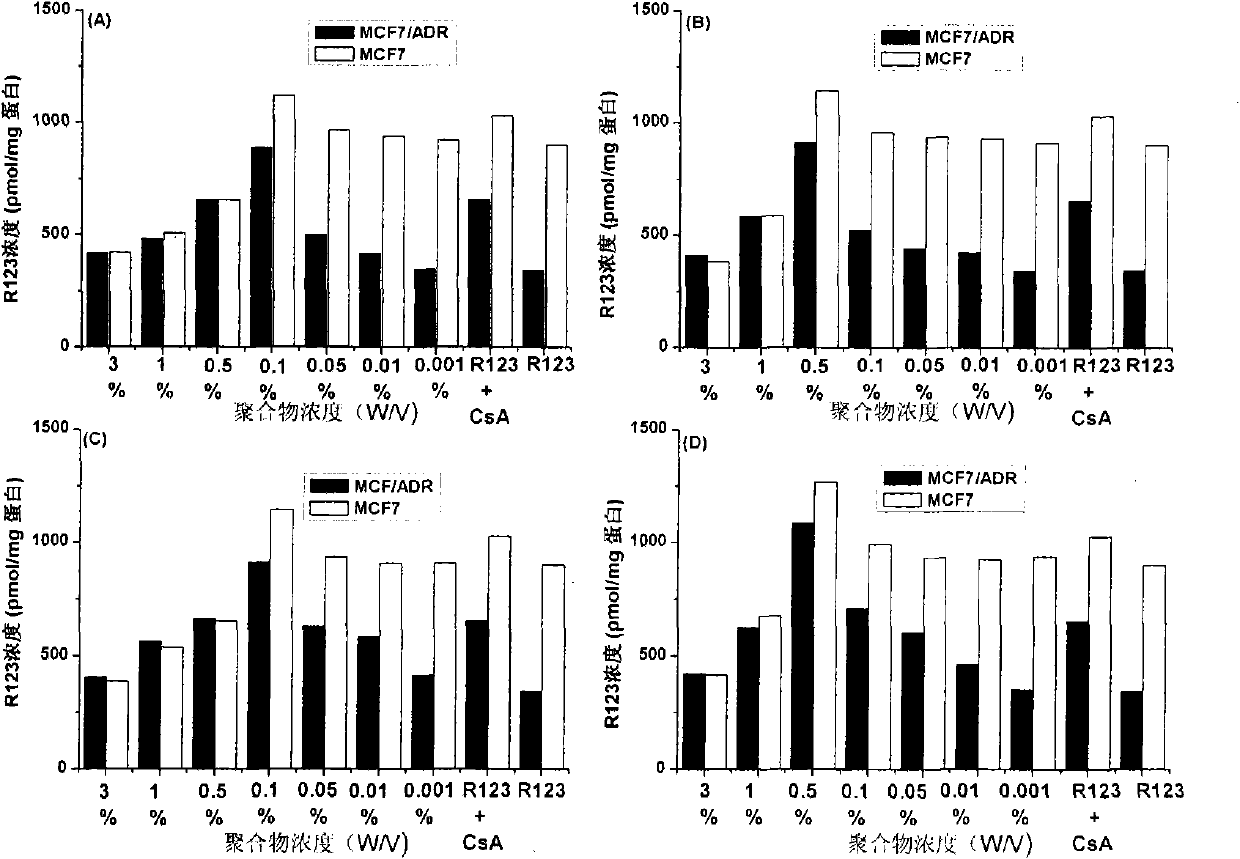
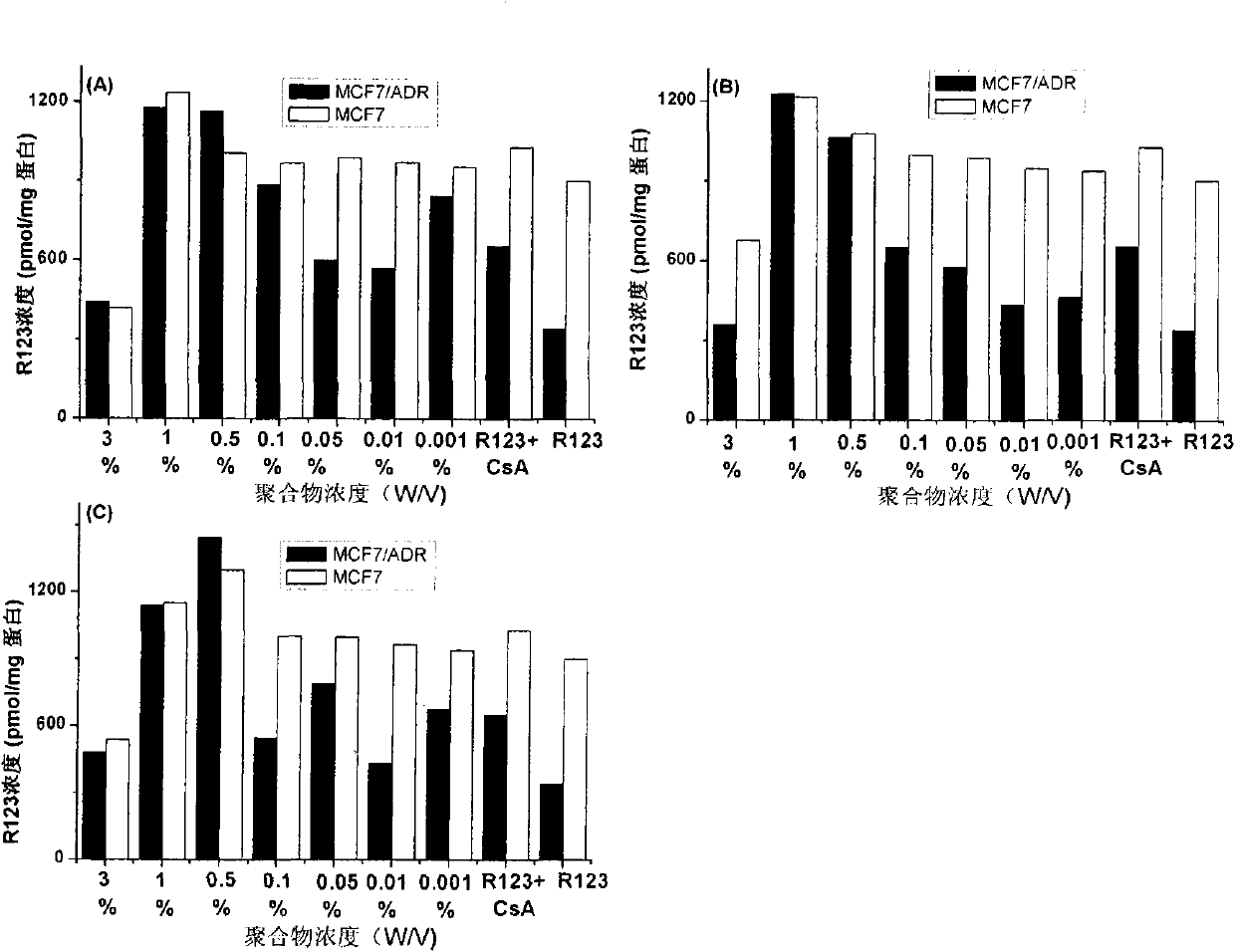
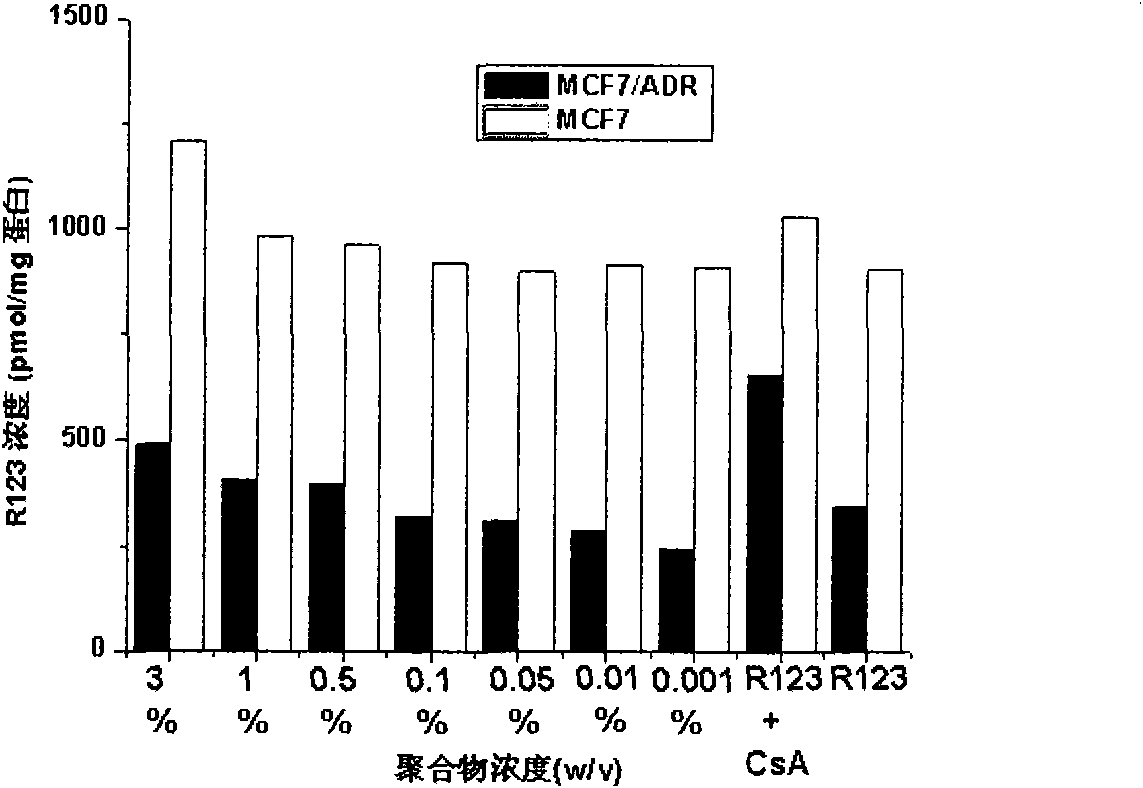
[0025] Example 1: Application of β-CD-PLA1000-mPEG2000 (Y: PLA, molecular weight 1000; Z: mPEG, molecular weight 2000) polymer micelles in inhibition of P-gp
[0026] 1. Preparation of β-CD-PLA1000-mPEG2000 polymer micelles and determination of their physical and chemical properties
[0027] Amino activation reaction:
[0028] Dissolve 11.3g (10mmol) of β-CD in 75ml of pyridine, slowly add 25ml of pyridine solution containing 13.3g (70mmol) of p-toluenesulfonyl chloride (TsCl) dropwise, and magnetically stir the reaction for 4 hours in an ice-water bath, then continue at room temperature Stir for 20h. After stopping the reaction, distill off most of the pyridine under reduced pressure at 40°C, soak in ether for 2 days, then form a fine powder, wash with water, filter, soak in acetone for 1 day, and dry in vacuum after filtering.
[0029] Add 5.0 g of sulfonylated β-CD into 20 ml of ethylenediamine, and react at 40° C. for 48 h. Concentrate the reaction solution under redu...
Embodiment 2
[0052] Example 2: Application of β-CD-PLA2000-mPEG2000 (Y: PLA, molecular weight 2000; Z: mPEG, molecular weight 2000) polymer micelles in inhibition of P-gp
[0053] 1. Preparation of β-CD-PLA2000-mPEG2000 polymer micelles and determination of their physical and chemical properties
[0054] Amino activation reaction: the method is the same as 1(1) of Example 1.
[0055] (2) Synthesis of hydroxyl-terminated Y-Z polymer:
[0056] Method is the same as 1(2) of Example 1. The dosage of mPEG2000 is 2g, the dosage of lactide is 2g, and the dosage of stannous octoate is 0.01g.
[0057] (3) Y-Z polymer terminal hydroxyl activation:
[0058] Method is the same as 1(3) of embodiment 1. mPEG2000-PLA2000:DMAP:TEA:succinic anhydride=1:1.2:1.2:1.2 (mol / mol).
[0059] (4) Cyclodextrin polymer synthesis:
[0060] Method is the same as 1(4) of Example 1. mPEG2000-PLA2000-COOH:DCC:DMAP:β-CDen7=1:7.2:7.2:7.2 (mol / mol). The NMR spectrum of the polymer is attached Figure 4 (B).
[00...
Embodiment 3
[0071] Example 3: Application of β-CD-PLA2000-mPEG5000 (Y: PLA, molecular weight 2000; Z: mPEG, molecular weight 5000) polymer micelles in inhibition of P-gp
[0072] 1. Preparation of β-CD-PLA2000-mPEG5000 polymer micelles and determination of their physical and chemical properties
[0073] Amino activation reaction: the method is the same as 1(1) of Example 1.
[0074] (2) Synthesis of hydroxyl-terminated Y-Z polymer:
[0075] Method is the same as 1(2) of Example 1. The dosage of mPEG5000 is 5g, the dosage of lactide is 2g, and the dosage of stannous octoate is 0.01g.
[0076] (3) Y-Z polymer terminal hydroxyl activation:
[0077] Method is the same as 1(3) of embodiment 1. mPEG5000-PLA2000:DMAP:TEA:succinic anhydride=1:1.2:1.2:1.2 (mol / mol).
[0078] (4) Cyclodextrin polymer synthesis:
[0079] Method is the same as 1(4) of Example 1. mPEG5000-PLA2000-COOH:DCC:DMAP:β-CDen7=1:7.2:7.2:7.2 (mol / mol). The NMR spectrum of the polymer is attached Figure 4 (C).
[00...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com