A sustained-release enteric-coated preparation of duloxetine, core material and preparation method thereof
A technology for duloxetine and enteric-coated preparations, which is applied in the field of duloxetine slow-release enteric-coated preparations and its core materials and preparations, which can solve problems such as adverse reactions and solvent residues, reduce solvent residues, and improve stability The effect of simplification and simplification of operation steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
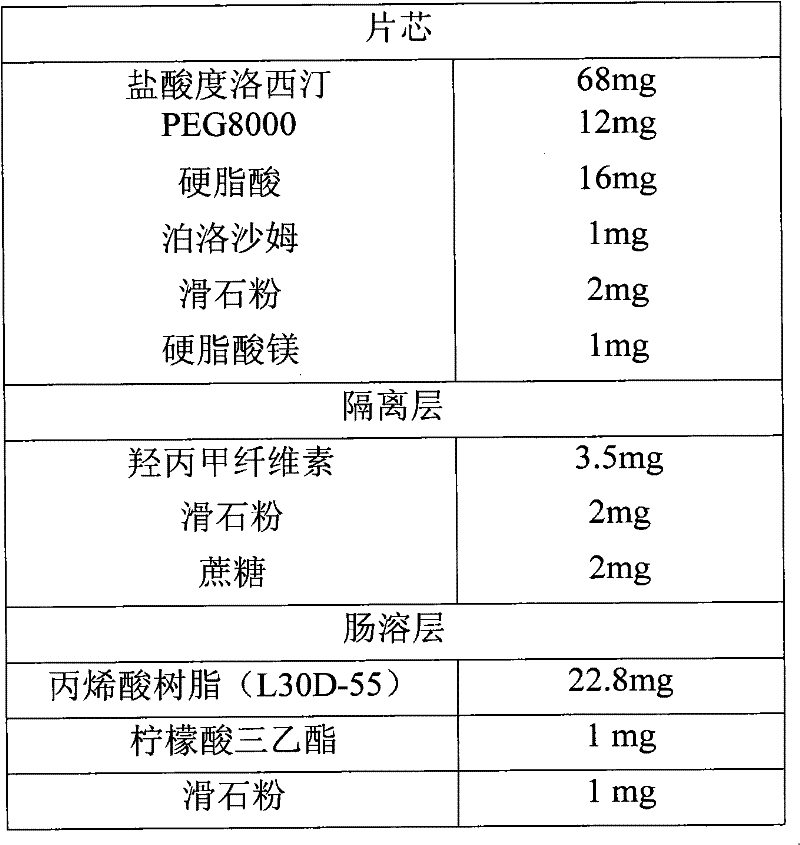
[0028] Example 1 Enteric-coated tablets
[0029] formula:
[0030]
[0031] Preparation Process:
[0032] Mix duloxetine hydrochloride, PEG8000, stearic acid and poloxamer, put it in a quick mixer with a jacket temperature of 82℃, turn on the mixer of the granulator, when the material temperature reaches 60℃, continue to stir to make 24 mesh Granules of different sizes are discharged, cooled, and granulated with a 24-mesh sieve. Add talcum powder and magnesium stearate, mix evenly, and press tablets to obtain tablet cores.
[0033] Isolation layer operation: In this example, duloxetine hydrochloride enteric-coated tablets were prepared. The coating preparation process is: disperse hypromellose with 70°C hot water, add water and stir to dissolve, add homogenized talc powder and Sucrose is made into a separation layer solution with a solid content of about 15% by weight. Put the tablet core in a coating pan to coat the isolation layer.
[0034] Enteric layer operation: add triethyl ci...
Embodiment 2
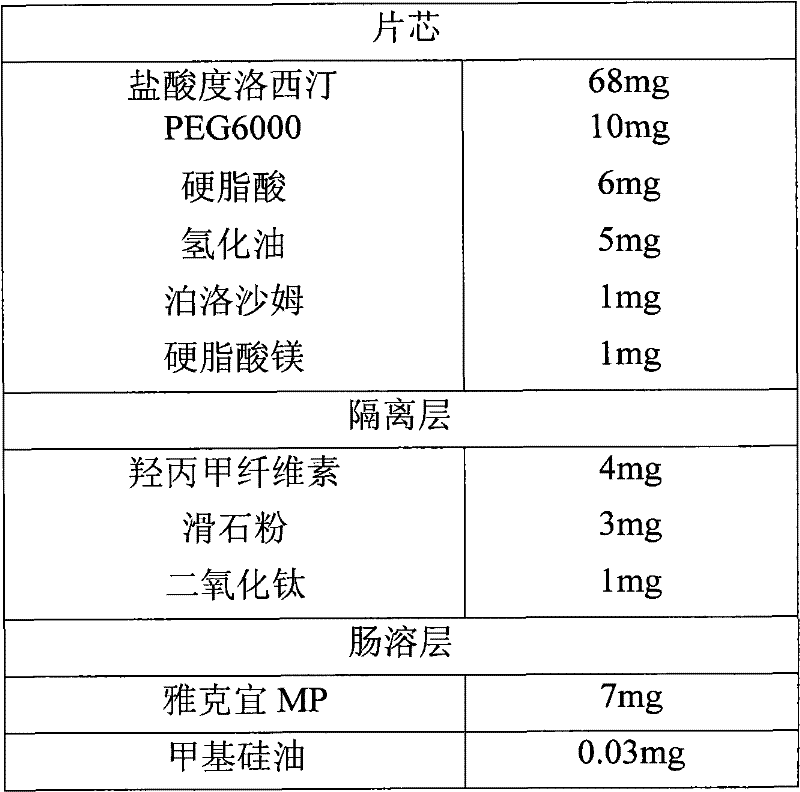
[0035] Example 2 Enteric-coated tablets
[0036] formula:
[0037]
[0038] Preparation Process:
[0039] Mix duloxetine hydrochloride, PEG6000, stearic acid, hydrogenated oil and poloxamer, put it in a quick mixer with a jacket temperature of 82℃, turn on the agitator of the granulator, when the material temperature reaches 60℃, continue to stir Into 24 mesh size particles, discharge, cool, and use a 24 mesh sieve to size. Add magnesium stearate, mix evenly, and press tablets to obtain tablet cores.
[0040] Isolation layer operation: In this example, duloxetine hydrochloride enteric-coated tablets were prepared. The coating preparation process is: disperse hypromellose with 70°C hot water, add water and stir to dissolve, add homogenized talc powder and Titanium dioxide is made into a separation layer solution with a solid content of about 15% by weight. Put the tablet core in a coating pan to coat the isolation layer.
[0041] Enteric layer coating: Acrylic MP is prepared as a 20w...
Embodiment 3
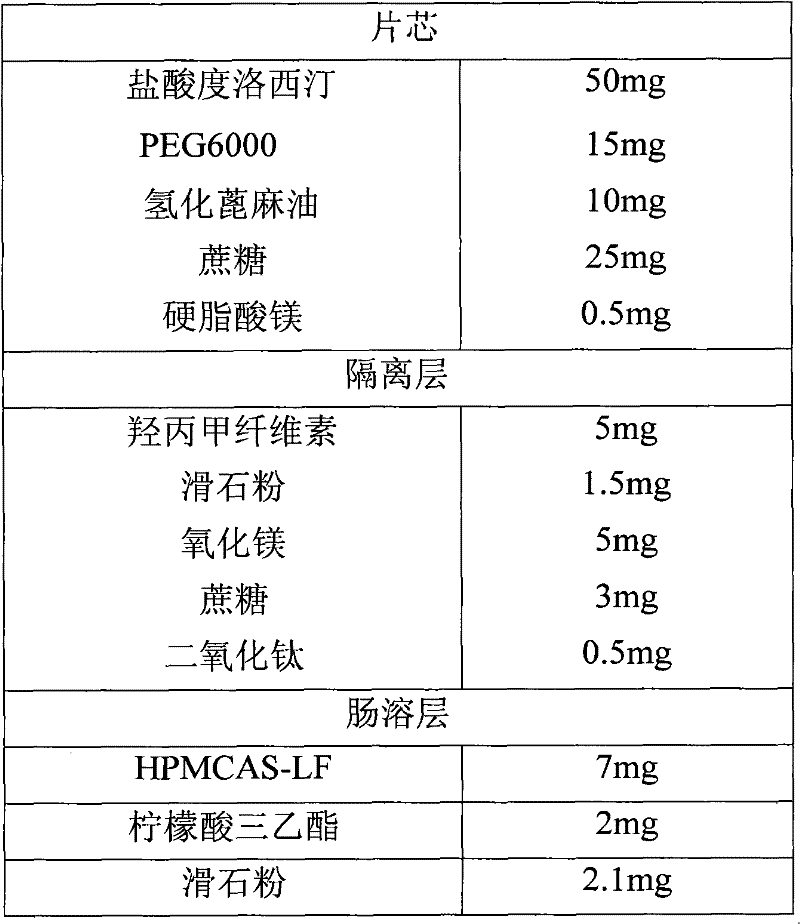
[0042] Example 3 Enteric-coated tablets
[0043] formula:
[0044]
[0045]
[0046] Preparation Process:
[0047] Mix duloxetine hydrochloride, PEG6000, hydrogenated castor oil and sucrose, put it in a quick mixer with a jacket temperature of 87℃, turn on the mixer of the granulator, when the material temperature reaches 65℃, continue to stir to make 24 mesh size The particles are discharged, cooled, and sized with a 24-mesh sieve. Add magnesium stearate, mix evenly, and press tablets to obtain tablet cores.
[0048] Isolation layer coating: Disperse hypromellose with 70℃ hot water and stir to dissolve it with water; take half of the solution, add homogenized talc, titanium dioxide and sucrose to make a solution with a solid content of about 15% by weight. The core is placed in a coating pan for coating; then magnesium oxide is added to the remaining half of the hypromellose solution to make a solution with a solid content of about 10 wt% for coating.
[0049] Enteric layer coating:...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com