Solid preparation of clopidogrel free alkali and preparation process thereof
A technology of clopidogrel free base and solid preparation, applied in the field of solid preparation of clopidogrel free base and its preparation, to achieve the effect of improving safety, stability and effective therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
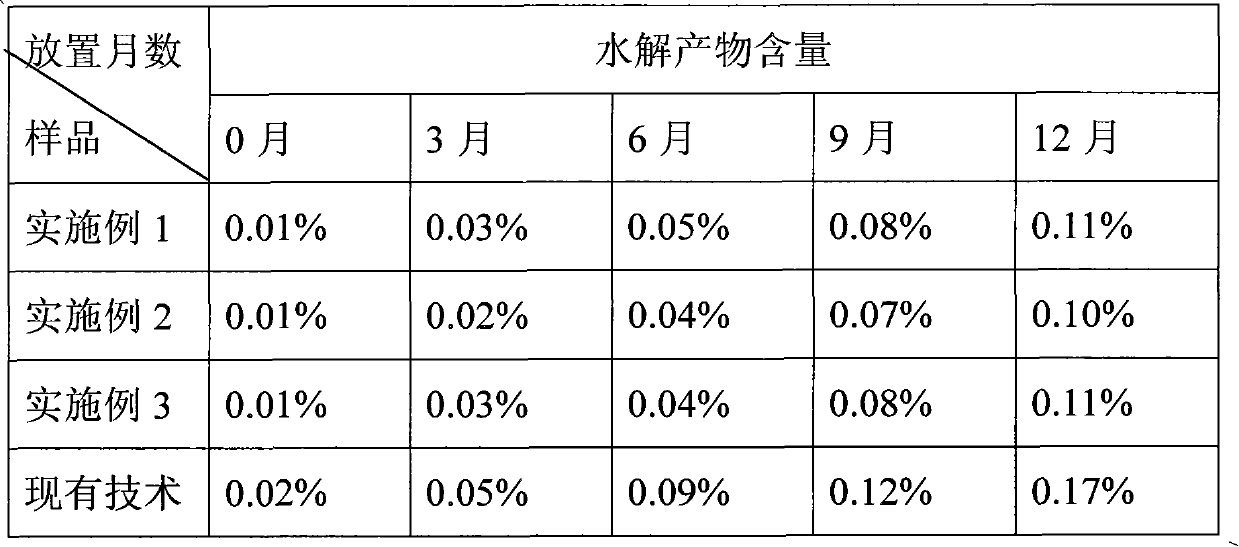
Examples
preparation example Construction
[0013] The preparation method of the solid preparation of the above-mentioned clopidogrel free base, the method is prepared by wet granulation, and the specific steps are as follows:
[0014] 1) Mix anhydrous lactose, microcrystalline cellulose, mannitol, and hydroxypropyl cellulose evenly, add clopidogrel free alkali solution for wet granulation, fluidize boiling drying, and sieve the granules through a high-speed crushing and sizing machine with a size of 1.0mm grain.
[0015] 2) Mix the granules obtained in operation 1) with part of the pregelatinized starch, fumed silicon dioxide, and magnesium stearate, and then make a solid preparation through a suitable method.
[0016] In the above operation 1), in order to meet the uniformity of clopidogrel free base, fully stirring and cutting in a high-speed wet granulator is adopted. In order to meet the uniformity of particle size, use a high-speed crushing and sizing machine with a 1.0mm sieve for sizing.
[0017] In the above-...
Embodiment 1
[0019] Tablet prescription with unit tablet weight 0.500g, clopidogrel labeled content 75mg / tablet and wet granulation preparation process
[0020] prescription
quantity
content%
Clopidogrel free base
75g
15%
120g
24%
microcrystalline cellulose
200g
40%
[0021] prescription
quantity
content%
20g
4%
20g
4%
partially pregelatinized starch
50g
10%
10g
2%
5g
1%
Total
500g
100%
[0022] 1) Mix 120g of anhydrous lactose, 200g of microcrystalline cellulose, 20g of mannitol, and 20g of hydroxypropyl cellulose, and add clopidogrel free base (75g) solution for wet granulation, fluidized boiling drying, and granulation by high-speed crushing Granulator 1.0mm sieve for granulation.
[0023] 2) Mix 343g of th...
Embodiment 2
[0025] Tablet prescription with unit tablet weight 0.420g, clopidogrel labeled content 75mg / tablet and wet granulation preparation process
[0026] prescription
quantity
content%
Clopidogrel free base
75g
17.9%
anhydrous lactose
123g
29.3%
140g
33.3%
15g
3.6%
15g
3.6%
partially pregelatinized starch
40g
9.5%
8g
1.9%
Magnesium stearate
4g
0.9%
Total
420g
100%
[0027] 1) Mix 123g of anhydrous lactose, 140g of microcrystalline cellulose, 15g of mannitol, and 15g of hydroxypropyl cellulose, add clopidogrel free base (75g) solution for wet granulation, fluidize boiling drying, and granulate through high-speed pulverization Granulator 1.0mm sieve for granulation.
[0028] 2) Mix 374g of the granules obtained in operation 1) with 40g of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com