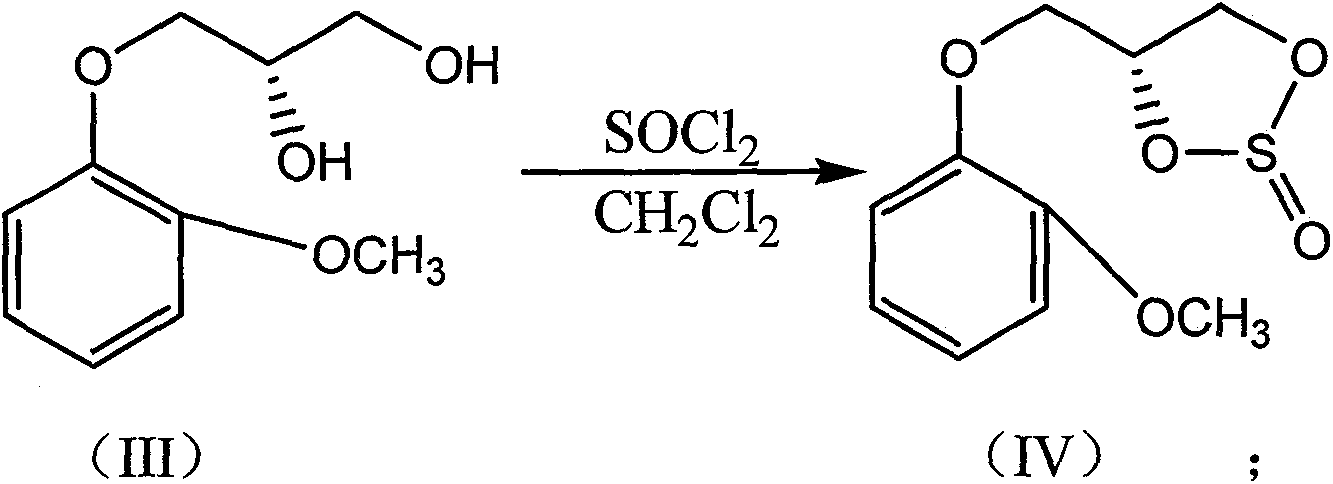
A kind of synthetic method of (s)-moprorol
A synthesis method and a technology of formula, applied in the synthesis field of moprol, can solve problems such as unfavorable industrial application, low optical purity, complicated operation, etc., and achieve good industrial application prospect, high optical purity and yield, and easy operation. simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040]Embodiment 1: the preparation of (S)-moprorol [compound 4 or formula (I)]
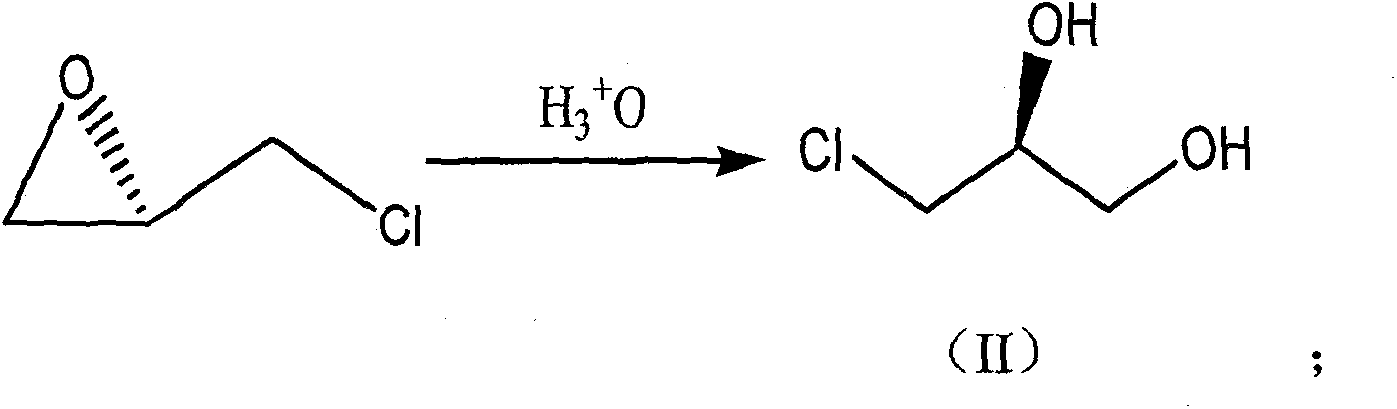
[0041] (1) Preparation of compound 1 or formula (II) [(S)-3-chloro-1,2-propanediol]
[0042] The temperature of 2wt.% dilute sulfuric acid (120g, 0.024mol) was slowly raised to 80°C, compound 1 (160mL, 2.043mol) was added dropwise, and the temperature was controlled at 100-105°C for 3 hours. Cool to room temperature, adjust the pH value to 7 with a 30wt.% sodium hydroxide solution in a water bath, concentrate under reduced pressure to remove water, and then distill under reduced pressure to collect fractions at 130-136°C / 2.67KPa to obtain a colorless and viscous Liquid (S)-3-chloro-1,2-propanediol 168g, yield 76.5%, experimental data are as follows:
[0043] Gas chromatography content: 99.4% (analysis conditions: AT SE-30 capillary column with column length 30m and diameter 0.25mm; hydrogen flame detection; column temperature: 180°C, monitor temperature: 230°C; carrier gas is N 2 , flow rate: 3...
Embodiment 2
[0054] (1) Preparation of compound 1 or formula (II) [(S)-3-chloro-1,2-propanediol]
[0055] The temperature of 1.5wt.% dilute sulfuric acid (80g, 0.012mol) was slowly raised to 80°C, compound 1 (160mL, 2.043mol) was added dropwise, and the temperature was controlled at 100-105°C for 3 hours. Cool to room temperature, adjust the pH value to 7 with a 30wt.% potassium hydroxide solution in a water bath, concentrate under reduced pressure to remove water, and then distill under reduced pressure to collect fractions at 130-136°C / 2.67KPa to obtain a colorless and viscous product Liquid (S)-3-chloro-1,2-propanediol 162g, yield 73.8%, GC content: 99.4%, [α] D 20 =+7.3(c 1.0, H 2 O).
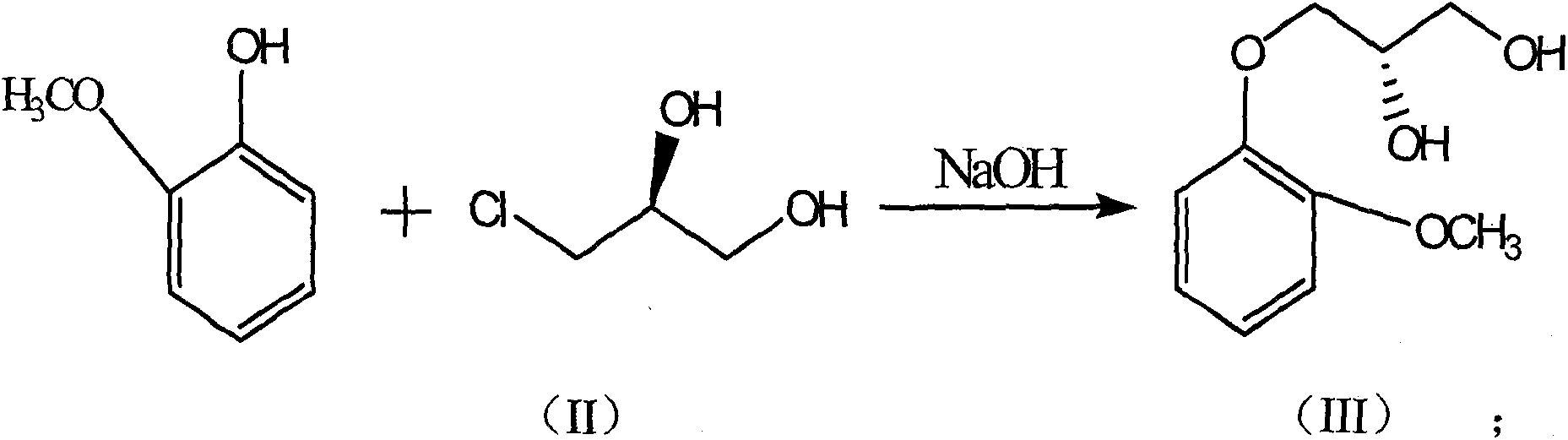
[0056] (2) Preparation of compound 2 or formula (III) (S)-guaifenesin [(S)-3-o-methoxyphenoxy-1,2-propanediol]
[0057] Guaiacol (12.4g, 0.1mol) was dissolved in 100mL of anhydrous methanol, sodium hydroxide (8.0g, 0.2mol) was added in batches at room temperature, and then a phase transfer catalyst ...
Embodiment 3
[0063] (1) Preparation of compound 1 or formula (II) [(S)-3-chloro-1,2-propanediol]
[0064] The concentration of 1wt.% dilute sulfuric acid (150g, 0.015mol) was slowly heated to 80°C, compound 1 (160mL, 2.043mol) was added dropwise, and the temperature was controlled at 100-105°C for 2 hours. Cool to room temperature, adjust the pH value to 7 with a 30wt.% sodium carbonate solution in a water bath, concentrate under reduced pressure to remove water, and then distill under reduced pressure to collect fractions at 130-136°C / 2.67KPa to obtain a colorless viscous liquid (S)-3-Chloro-1,2-propanediol 172g, yield 78.3%, gas chromatography content: 99.6%, [α] D 20 =+7.4(c 1.0, H 2 O).
[0065] (2) Preparation of compound 2 or formula (III) (S)-guaifenesin [(S)-3-o-methoxyphenoxy-1,2-propanediol]
[0066] Guaiacol (12.4g, 0.1mol) was dissolved in 100mL of isopropanol, sodium hydroxide (7.0g, 0.18mol) was added in batches at room temperature, and then a phase transfer catalyst benz...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com