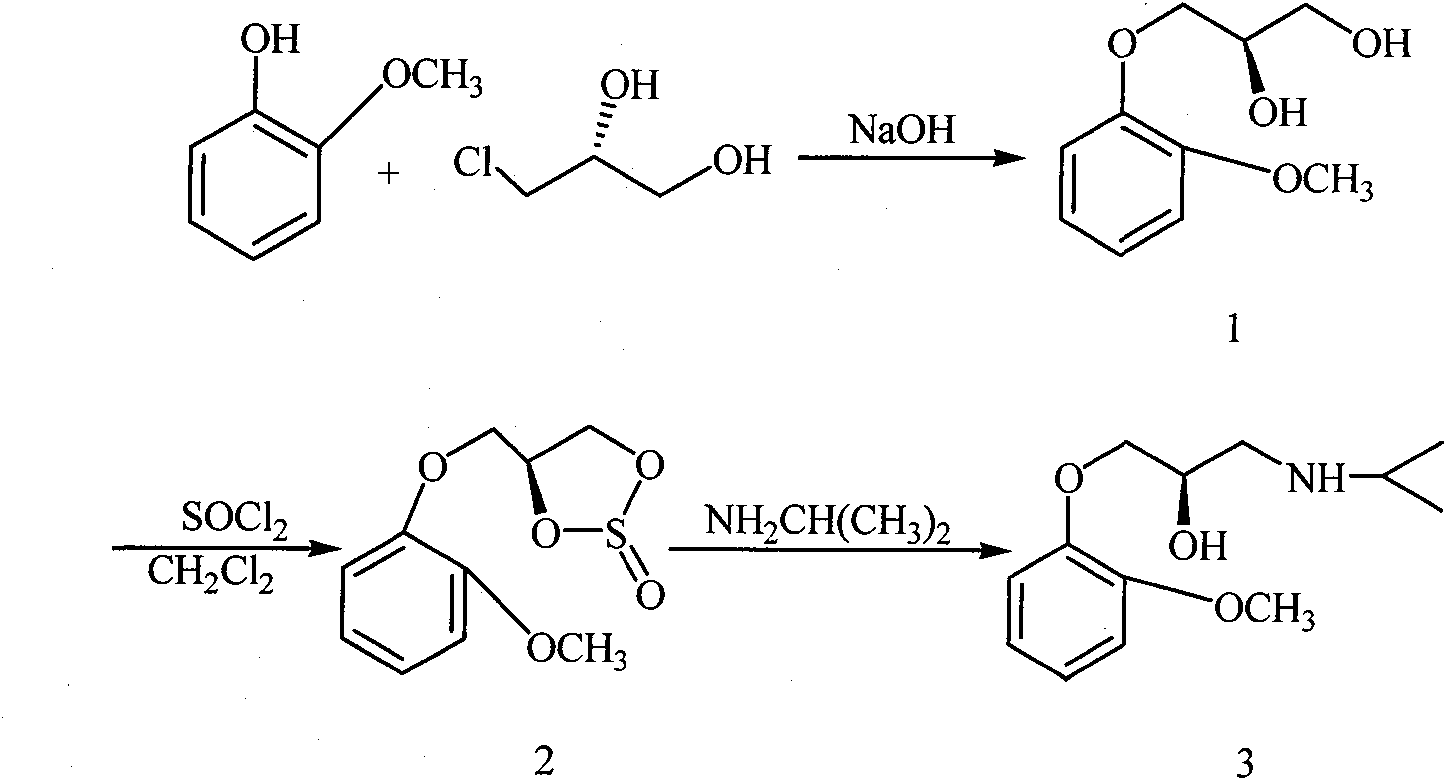
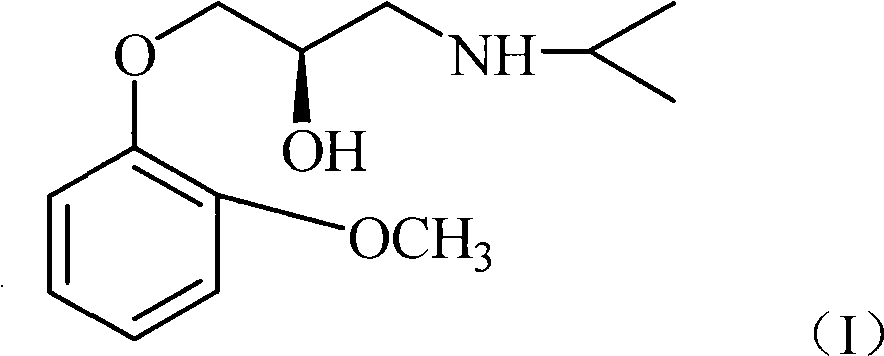
Synthesis method of (R)-moprolol
A synthesis method and a technology of the equation, applied in the synthesis field of moprol, can solve the problems of need, not suitable for industrial application, expensive reagents, etc., and achieve the effects of simple operation, good industrial application prospect and short cycle.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: the preparation of (R)-moprorol [compound 3 or formula (I)]
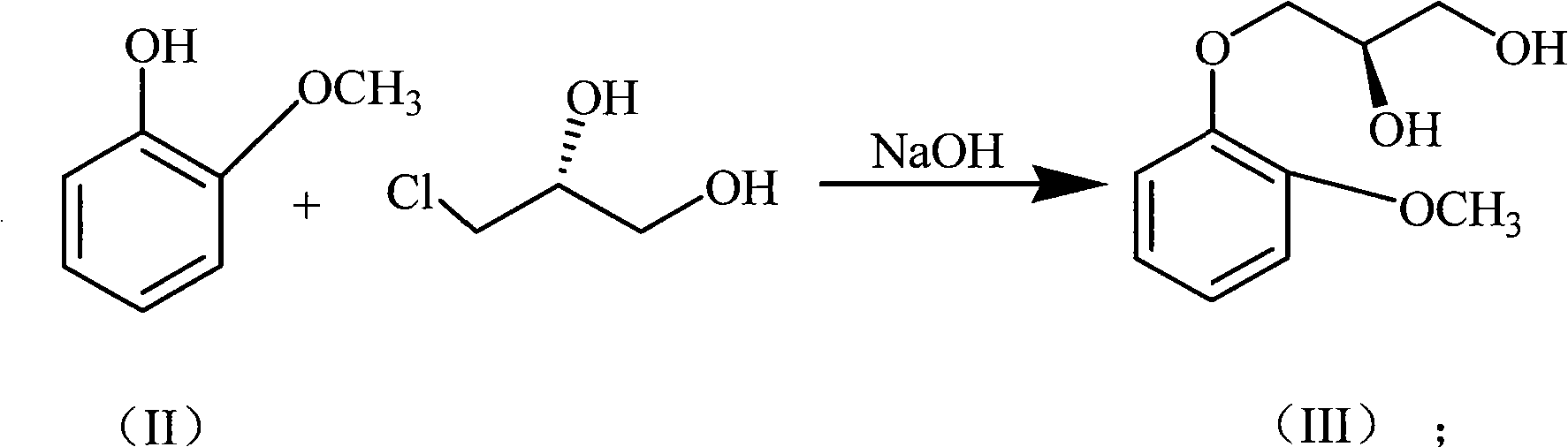
[0033] (1) Preparation of compound 1 or formula (III) [(R)-3-o-methoxyphenoxy-1,2-propanediol]
[0034] Dissolve guaiacol (29.8, 0.24mol) in 240mL of absolute ethanol, add sodium hydroxide (10.7g, 0.27mol) in batches at room temperature, then add phase transfer catalyst tetrabutylammonium bromide (0.32g, 1.0mmol), add (R)-3-chloro-1,2-propanediol (23.3g, 0.21mol) dropwise, after dropping, react at 70-73°C for 5 hours, after the reaction is completed, filter while hot, and concentrate the filtrate under reduced pressure, Petroleum ether was recrystallized to obtain 33.2 g of white solid (R)-guaifenesin, with a yield of 80.1%. The experimental data are as follows:
[0035] mp 97-99°C, [α] D 20 = -9.5 (c 1.0, MeOH); 1 HNMR (400MHZ, CDCl 3 ): δ3.77-3.83 (m, 2H, CH 2 ), 3.86(s, 3H, CH 3 ), 4.06-4.09 (m, 2H, CH 2 ), 4.16-4.20 (m, 1H, CH), 6.89-7.01 (m, 4H, Ar).; IR (KBr) cm -1 : 3242, 2941, 2...
Embodiment 2
[0043] (1) Preparation of compound 1 or formula (III) [(R)-3-o-methoxyphenoxy-1,2-propanediol]
[0044] Guaiacol (14.9g, 0.12mol) was dissolved in 120mL of anhydrous methanol, sodium hydroxide (8.0g, 0.2mol) was added in batches at room temperature, and then a phase transfer catalyst benzyltriethylammonium chloride (benzyltriethylammonium) was added ( 0.24g, 1.0mmol), drop (R)-3-chloro-1,2-propanediol (23.3g, 0.21mol), dropwise, 55 ~ 60 ℃ for 8 hours, the reaction is complete, filtered while hot, the filtrate reduced Concentrated under reduced pressure, recrystallized from toluene to obtain 19.2 g of white solid (R)-guaifenesin, yield 81.6%, mp97-99°C, [α] D 20 = -9.4 (c 1.0, MeOH)
[0045] (2) Preparation of compound 2 or formula (IV) [(S)-4-o-methoxyphenoxymethyl-1,3,2-dioxathiolane-2-oxide]
[0046] Compound 1 (9.9 g, 0.05 mol) was dissolved in 100 mL of dichloromethane, and a mixed solution of thionyl chloride (6.5 g, 0.055 mol) and 10 mL of dichloromethane was added dr...
Embodiment 3
[0050] (1) Preparation of compound 1 or formula (III) [(R)-3-o-methoxyphenoxy-1,2-propanediol]
[0051] Dissolve guaiacol (12.4g, 0.1mol) in 240mL isopropanol, add sodium hydroxide (6.0g, 0.15mol) in batches at room temperature, then add phase transfer catalyst tetrabutylammonium bromide (0.16g , 0.5mmol), dropwise added (R)-3-chloro-1,2-propanediol (44.3g, 0.4mol), after dropping, reacted at 80-85°C for 4 hours, after the reaction was completed, filtered while hot, and concentrated the filtrate under reduced pressure , recrystallized from cyclohexane to obtain 17.3 g of white solid (R)-guaifenesin, yield 87.3%, mp97-99°C, [α] D 20 = -9.4 (c 1.0, MeOH)
[0052] (2) Preparation of compound 2 or formula (IV) [(S)-4-o-methoxyphenoxymethyl-1,3,2-dioxathiolane-2-oxide]
[0053] Compound 1 (10.0 g, 0.05 mol) was dissolved in 100 mL of dichloromethane, and a mixed solution of thionyl chloride (6.5 g, 0.055 mol) and 10 mL of dichloromethane was added dropwise at a temperature contr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com