Novel chalcone compound and preparation method and application thereof
A technology for chalcone and compound, which is applied in the field of chalcone compound and its preparation, can solve the problems of lack of scientific basis for clinical drug use, complex chemical composition of Pseudomonas falciparum, difficulty in formulating quality standards, etc., and achieves reasonable design of process route. , Single ingredient, strong inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
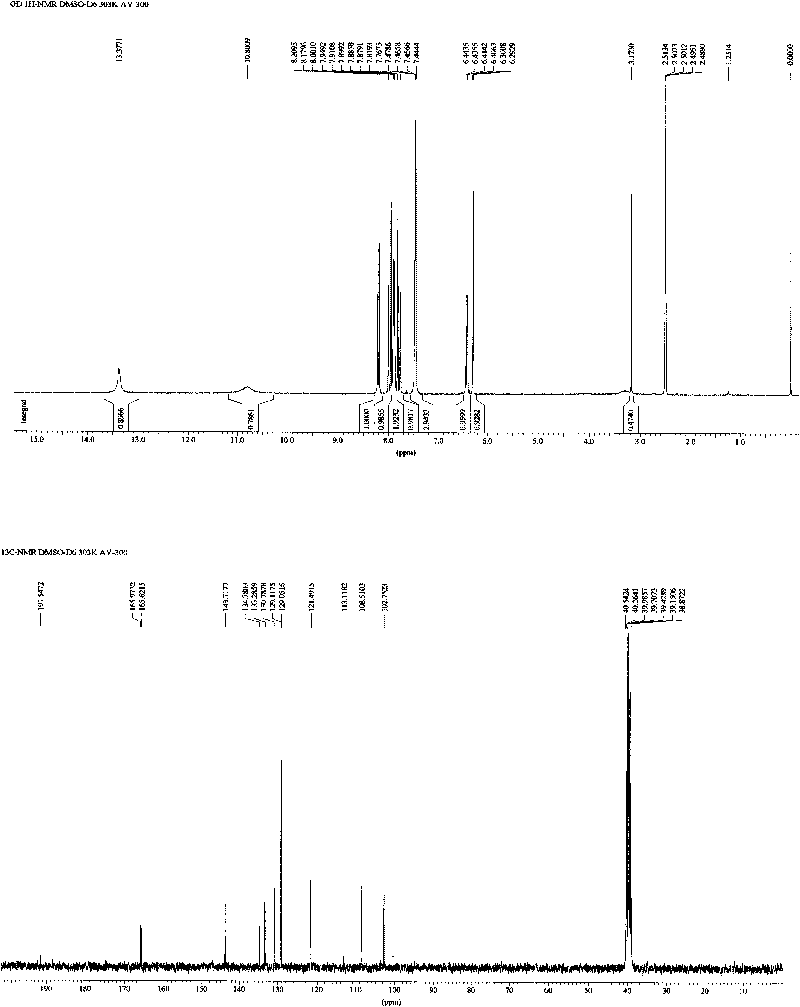
[0031] The preparation of embodiment 1 new chalcone compound:
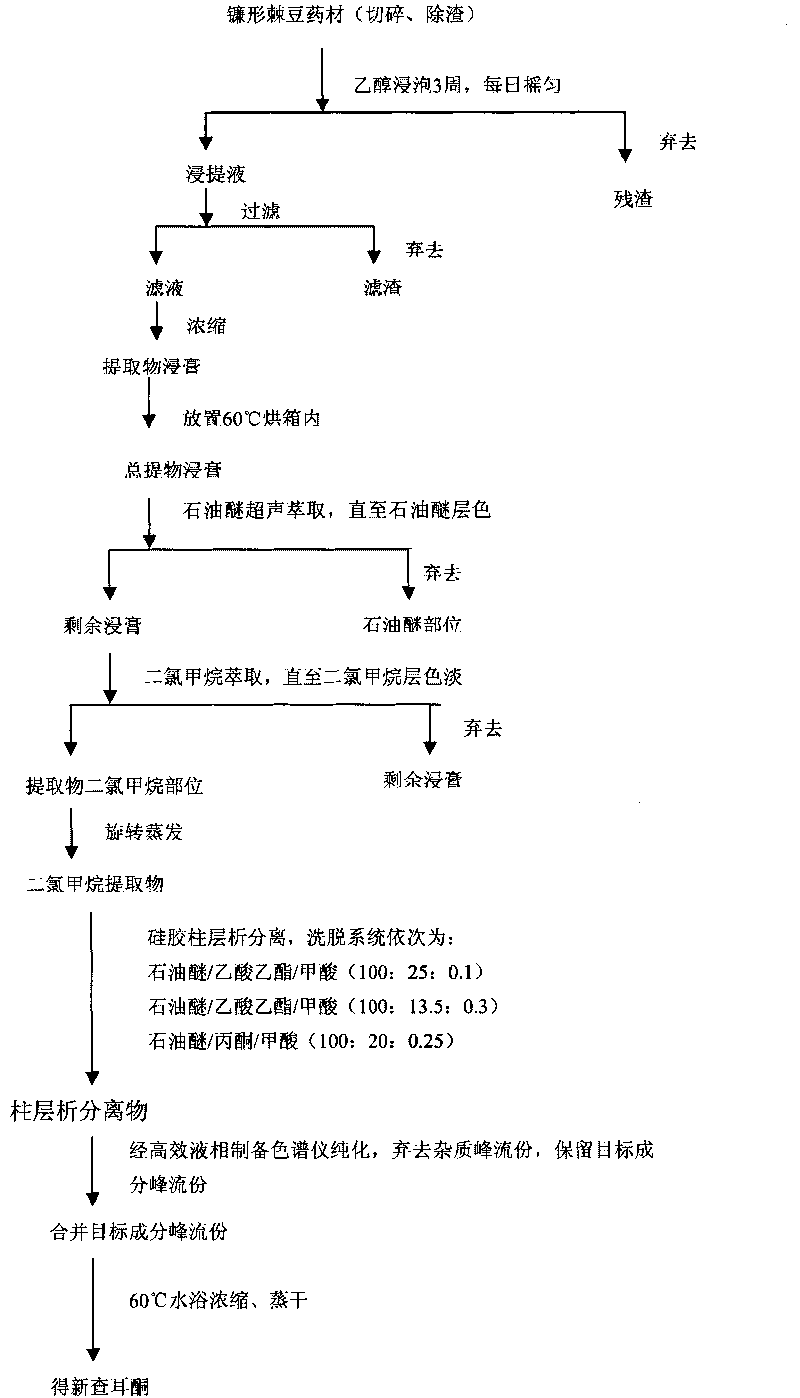
[0032] Such as figure 1 Shown, the preparation method of the new chalcone compound provided by the invention comprises the following steps:
[0033] (1) Take 500g of Oxytropis falciparum dried medicinal material, chop, remove residue, soak with 95% ethanol for 3 weeks, and shake every day; after 3 weeks, take out the extract, filter, and the filtrate is decompressed by a rotary evaporator Concentrate to extract, set aside;
[0034] (2) Put the extract obtained in step (1) into a 500ml beaker, add 200ml of petroleum ether (60-90°C) for ultrasonic extraction, replace the petroleum ether every 20min, and the color of petroleum ether becomes light after extraction for 2 hours, discard the petroleum Ether extract, keep the remaining extract; add 200ml of dichloromethane to the remaining extract for ultrasonic extraction, replace the dichloromethane every 20min, after extraction for 3h, combine all the collected dichl...
Embodiment 2
[0042] The preparation of embodiment 2 new chalcone compounds:
[0043] Such as figure 1 Shown, the preparation method of the new chalcone compound provided by the invention comprises the following steps:
[0044] (1) Take 500g of Oxytropis falciparum dried medicinal material, chop, remove residue, soak with 90% ethanol for 4 weeks, and shake every day; after 4 weeks, take out the extract, filter, and the filtrate is decompressed by a rotary evaporator Concentrate to extract, set aside;
[0045] (2) Put the extract obtained in step (1) into a 500ml beaker, add 200ml of petroleum ether (60-90°C) for ultrasonic extraction, replace the petroleum ether every 20min, and the color of petroleum ether becomes light after extraction for 2 hours, discard the petroleum Ether extract, keep the remaining extract; add 200ml of dichloromethane to the remaining extract for ultrasonic extraction, replace the dichloromethane every 20min, after changing 6 times, combine all the collected dichl...
Embodiment 3
[0053] Example 3 Anti-tumor experimental research on new chalcones
[0054] 1. Preparation of the test drug: Take an appropriate amount of the new chalcone compound monomer provided by the invention, dissolve it with dimethyl sulfoxide (DMSO), and prepare the concentration of 1.25 μg, 2.5 μg, 5 μg, and 10 μg respectively. , 20 μg of the test drug, the final concentration of DMSO was 1.0%, after filter sterilization, it was stored at 4°C for later use, and dimethyl sulfoxide was used as the test control group.
[0055] 2. Experimental process
[0056] Human liver cancer cell line SMMC-7721, human cervical cancer cell line Hela, human gastric cancer cell line MGC-803, human colon cancer cell line LOVO, human breast cancer cell line MDA-MB-231, and human lung cancer cell line A549 were selected. Liver cancer cell line SMMC-7721, human breast cancer cell line MDA-MB-231 and human colon cancer cell line LOVO cell line were cultured in RPMI-1640 medium containing 10% calf serum. Hu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com