Compound slow-release preparation of benorilate, pseudoephedrine and chlorphenamine
A technology of pseudoephedrine and slow-release preparations, which is applied in the field of medicine to achieve the effects of high safety, less frequency of medication, and reduced frequency of taking
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
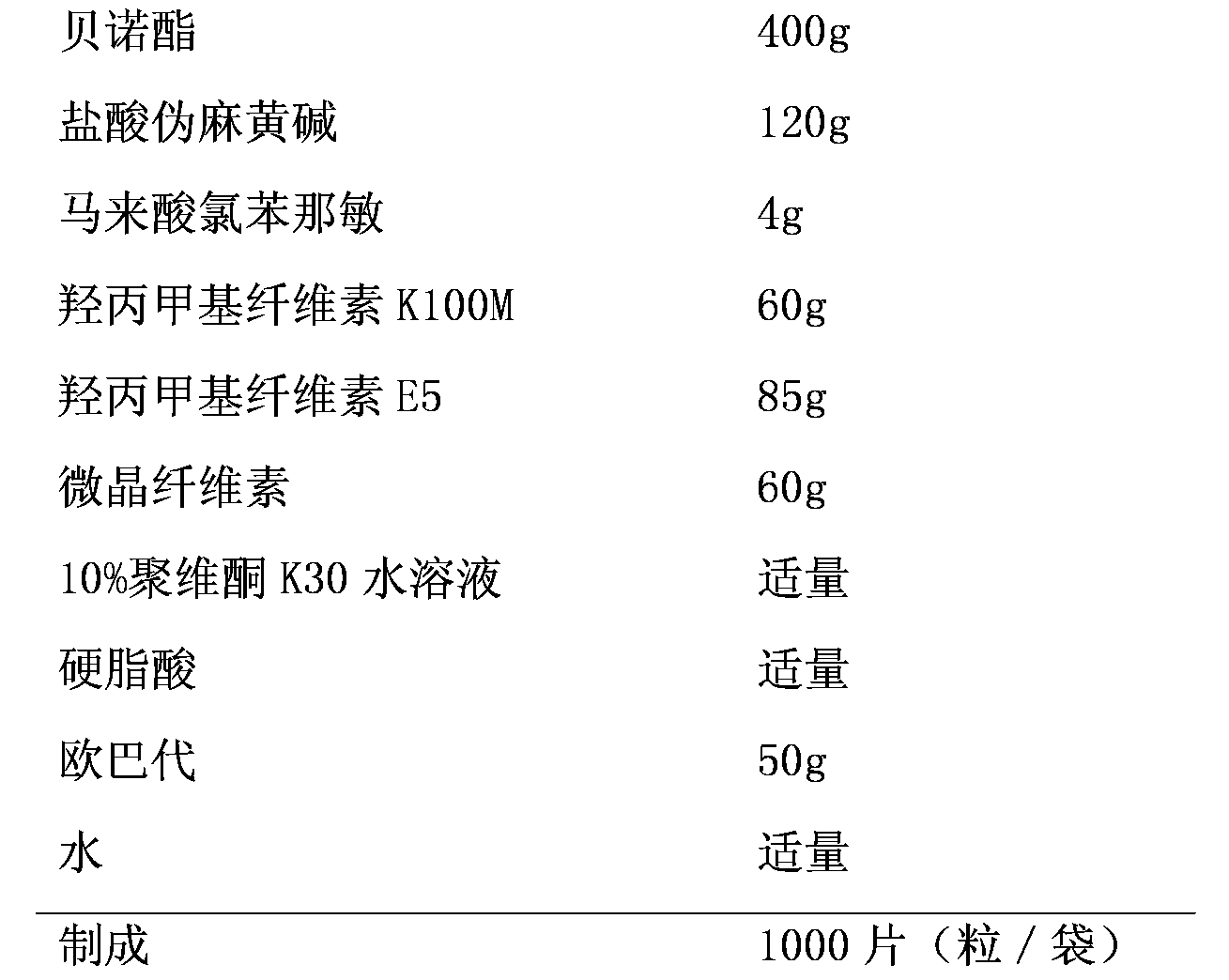
[0020] prescription:
[0021]
[0022] Preparation method 1:
[0023] (1) Preparation of granules Hypromellose and microcrystalline cellulose were sieved separately and mixed evenly. Then add pseudoephedrine hydrochloride, chlorpheniramine maleate, and benoxylate in sequence, mix well, use 5% povidone K30 aqueous solution as a binder to make a soft material, make wet granules with a 20-mesh sieve, dry at 50°C, and dry for 20 Mesh sieve whole grain, set aside.
[0024] (2) Preparation of coating solution Add Opadry to pure water, and add pure water to 100ml, stir for 1 hour, set aside.
[0025] (3) Coating the granules of (1) to obtain coated granules.
[0026] (4) Add an appropriate amount of magnesium stearate to the granules obtained in (3), mix well, and press into tablets to obtain tablets.
[0027] (5) Add an appropriate amount of magnesium stearate to the granules obtained in (1), mix well, press into tablets, take (2) coating solution, wrap it on the outside of t...
Embodiment 2
[0039] prescription:
[0040]
[0041] Preparation method 1:
[0042](1) Preparation of granules: sieve hypromellose and microcrystalline cellulose separately, and mix well by equal increment method, then add pseudoephedrine hydrochloride, beinolate and chlorpheniramine maleate in the prescribed amount 2g, make it mix evenly, use 10% copovidone K30 aqueous solution as binder to make soft material, make wet granules with 20 mesh sieve, dry at 60°C, granulate with 20 mesh sieve, and set aside.
[0043] (2) Preparation of coating solution Add 2 g of chlorpheniramine maleate and Opadry to pure water, and add pure water to 100 ml, stir for 0.5 hours, and set aside.
[0044] (3) Coating the granules of (1) to obtain coated granules.
[0045] (4) Add an appropriate amount of stearic acid to the granules obtained in (3), mix well, and press into tablets to obtain Tablet I.
[0046] (5) Add an appropriate amount of stearic acid to the granules obtained in (1), mix well, press int...
Embodiment 3
[0060] prescription:
[0061] pseudoephedrine sulfate
120g
4.0 g
Benoate
400g
blank core
120g
Hypromellose E5
10g
Surelease Solids
30g
10g
Titanium dioxide
1g
5g
Povidone K 30
Appropriate amount
water
Appropriate amount
Absolute ethanol
Appropriate amount
[0062] Coating prescription:
[0063] Opadry
30 g
water
Add to 1000ml
[0064] Made into 1000 grains (tablets)
[0065] Preparation:
[0066] (1) Pseudoephedrine Sulfate Sustained Release Pellets
[0067] Ⅰ. Prepare 10% HPMC E5 aqueous solution for later use;
[0068] Ⅱ. Take the Surelease solid, add water, shake well and set aside;
[0069] Ⅲ. Prepare a water-soluble coating solution containing 10% of water-soluble coating powder and 1% of titanium dioxide;
[0070] Ⅳ, prepare 40...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com