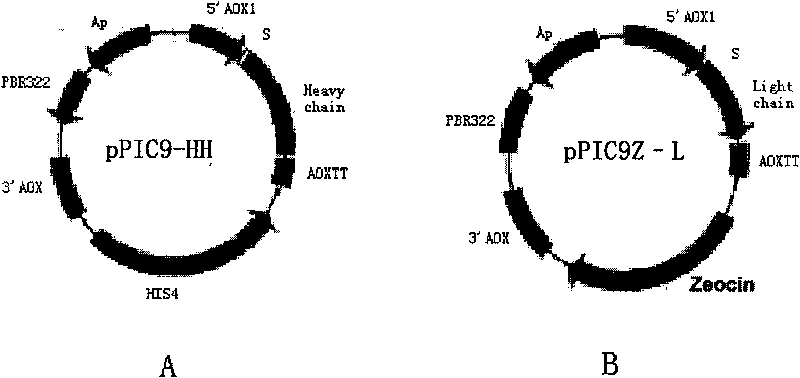
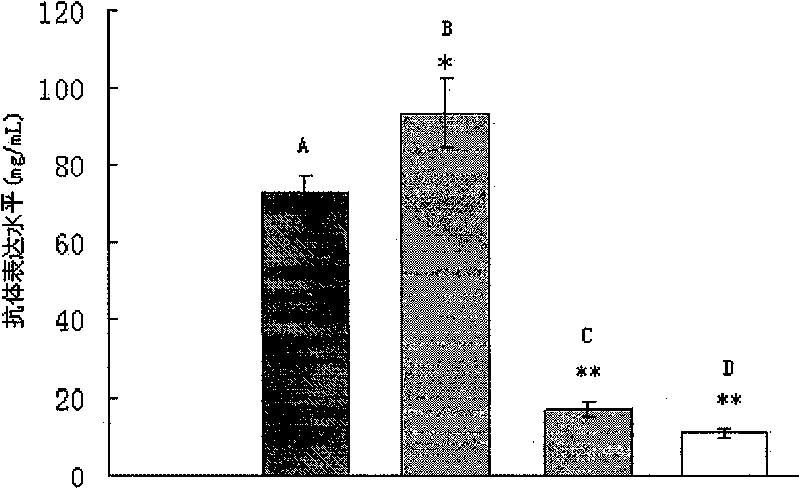
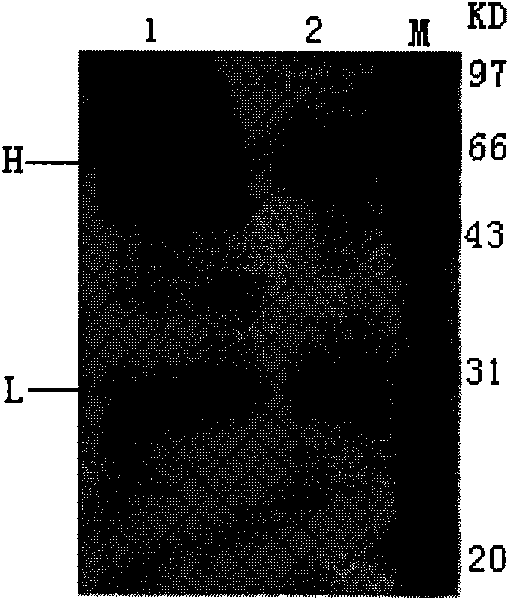Recombinant yeast for expressing antibody or antibody analogue as well as construction method and application thereof
An antibody analog, yeast technology, applied in microorganism-based methods, biochemical equipment and methods, recombinant DNA technology, etc., can solve the problems of high antibody drug treatment, high culture cost, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1: Expression of anti-HER2 humanized monoclonal antibody in Pichia pastoris
[0039] 1. Obtaining the whole gene of anti-HER2 humanized monoclonal antibody
[0040] According to the light and heavy chain sequences of anti-HER2 humanized antibodies reported on Genbank (Genbank: AAB49171.4, and AAB48814.1) and the preferred codons of Pichia pastoris, the genes for the light and heavy chains were designed. The nucleotide sequence of the light chain is sequence 3 in the sequence listing, and the nucleotide sequence of the heavy chain is sequence 4 in the sequence listing.
[0041] Use the one-step PCR method to design primers, that is, the gene is divided into two sections, the first section is segmented to synthesize a 59bp forward fragment, and each segment overlaps by 20bp, and the latter segment is segmented to synthesize a 59bp reverse fragment. 1pmol, mixed, head-to-tail primers as forward and reverse primers, PCR amplified spliced gene to obtain light chai...
Embodiment 2
[0112] Example 2, Expression of anti-Her2 humanized antibody in Kluyveromyces lactis
[0113] 1. Acquisition of light chain and heavy chain genes of anti-HER2 humanized antibody containing different signal peptides
[0114] Using the vector pPIC9-HL constructed in Example 1, PCR amplifies the antibody heavy chain gene H (Heavy chain) fragment containing the HSA signal peptide, and the primer is HSA-KL-01 (5'-CG ACTAGT CAAACGATGAAGTGGGTAACCTTTATTTCCCTTCTTTTTCTCTTTAGCTCGGC-3', the underlined part is the SpeI restriction site) and primer Her2-03: (5'-CG GCGGCCGC TTACTTACCTGGAGACAAAGACAAAG-3', the underlined part is the NotI restriction site).
[0115] The PCR reaction conditions were: pre-denaturation at 94°C for 5 min; denaturation at 94°C for 30 sec, annealing at 60°C for 30 sec, extension at 72°C for 2 min, and a total of 30 cycles, with a final extension at 72°C for 10 min.
[0116] The PCR product was recovered by a DNA fragment purification recovery kit.
[0117] Using...
Embodiment 3
[0186] Example 3, Expression of Antibody Analog sTNFRII-IgGFc in Pichia pastoris
[0187] 1. sTNFRII-IgGFc fusion protein gene cloning
[0188]Take acid citrate dextrose (ACD) anticoagulant venous blood from healthy people, dilute it with Hanks solution, centrifuge with lymphocyte separation medium to obtain lymphocytes, adjust the number of cells with 1640 medium containing 10% fetal calf serum (Hyclone company) 5×10 6 cells / mL, placed in a cell incubator and incubated for 2 hours, replaced with lipopolysaccharide (LPS, 20 μg / mL) (Sigma Company) and 10% fetal bovine serum 1640 fresh medium, continued to cultivate for 5 hours, and centrifuged to enrich the cells; Total RNA was extracted by TRIzol (Shanghai Sangon Bioengineering Technology Service Co., Ltd.) method (operated according to the instructions of TRIzol); the total RNA was reverse-transcribed to obtain cDNA (RT-PCR kit, Shanghai Sangon Bioengineering Technology Service Co., Ltd., according to Kit instructions to op...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Relative molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com