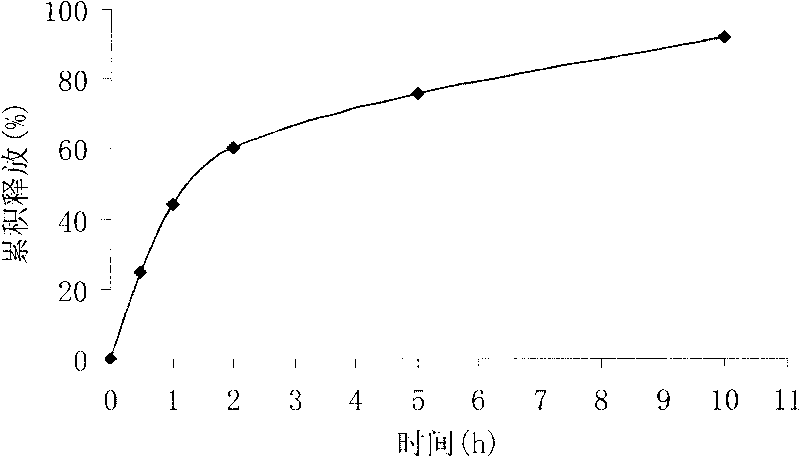
Quick-release tablet and preparation method thereof
An immediate-release tablet and immediate-release tablet technology, applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, oil/fat/wax non-active ingredients, etc., can solve the problem of no immediate-release tablets and no long-term dispersion In order to achieve the effects of increasing drug stability, a wide range of drug selection, and good therapeutic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0016] The preparation method of the immediate-release tablet of the present invention is to divide the original drug that is called the immediate-release tablet into two parts, and one part adopts pharmaceutically acceptable microencapsulation, coating method, hydrophobic solid dispersion or matrix type Granules are prepared into sustained-release drug components, and then another part of the original drug is prepared into immediate-release drug components by a pharmaceutically acceptable micronization method, micro-powder crystallization method, or hydrophilic solid dispersion method, and then mixed with sustained-release drug components. The ingredients are mixed separately, and finally prepared into immediate-release tablets by ordinary tablet technology.
[0017] The above disintegrants and / or fillers are selected from pharmaceutically acceptable disintegrators and / or fillers, including starch, pregelatinized starch, lactose, agar, croscarmellose sodium, carboxymethyl One...
Embodiment 1
[0025] Embodiment 1: Quinapril Hydrochloride Immediate Release Tablets
[0026] prescription:
[0027] Quinapril hydrochloride 4g (1g is raw material powder, 3g is wrapped into microcapsules)
[0028] Lactose 2g
[0029] Microcrystalline Cellulose 3g
[0030] Cross-linked polyvinylpyrrolidone 4g (add 2g inside; add 2g outside)
[0031] Sodium carboxymethyl starch 1g
[0032] Aspartame 0.6g
[0033] Magnesium stearate 0.15g
[0034] Micronized silica gel 0.1g
[0035] 10% polyvinylpyrrolidone K30 alcohol 3ml
[0036] A total of 100 pieces were made
[0037] Prepared by wet granulation and tabletting technology: quinapril hydrochloride micropowder, lactose, microcrystalline cellulose, PVPP (internal added part) are fully mixed and passed through a 60-mesh sieve, and 10% polyvinylpyrrolidone K30 alcohol solution is added to prepare Moderately wet and dry soft material, granulate with 20 mesh sieve, dry at 50°C for 3 hours, granulate with 20 mesh sieve, add quinapril micr...
Embodiment 2
[0040] Embodiment 2: Valacyclovir hydrochloride immediate release tablet
[0041] Each 100 tablets contains the following substances
[0042] Valacyclovir hydrochloride 15g (3g raw material micropowder, 12g encapsulated into microcapsules)
[0043] Lactose 2g
[0044] Microcrystalline Cellulose 3g
[0045] Cross-linked polyvinylpyrrolidone 4g (add 2g inside; add 2g outside)
[0046] Sodium carboxymethyl starch 1g
[0047] Aspartame 0.5g
[0048] Magnesium stearate 0.1g
[0049] Micronized silica gel 0.1g
[0050] Tween 800.3g
[0051] 5% polyvinylpyrrolidone S630 aqueous solution 10ml
[0052] A total of 100 pieces were made
[0053] Preparation process: valacyclovir hydrochloride micropowder, lactose, microcrystalline cellulose, and cross-linked polyvinylpyrrolidone (internal added part) are fully mixed and passed through a 60-mesh sieve, and an aqueous solution containing Tween 80 and 5% polyvinylpyrrolidone S630 is added, Make a soft material with moderate dryness a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com