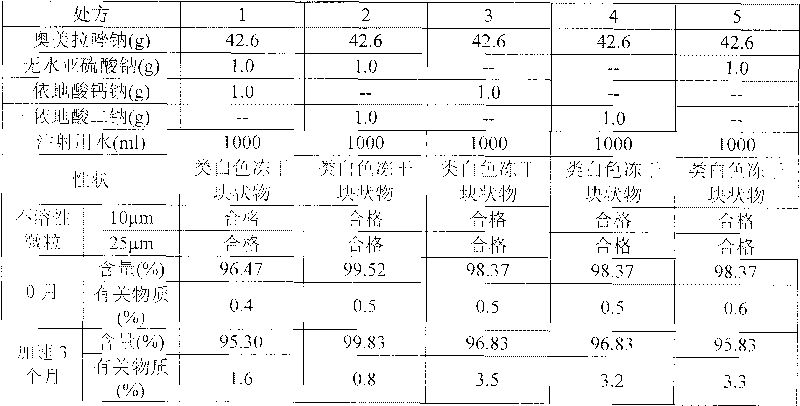Omeprazole sodium freeze-dried powder injection, as well as preparation method and quality control method thereof
A technology of omeprazole sodium and freeze-dried powder injection, which is applied in the field of medicine and can solve the problems of reduced drug efficacy, increased side effects, and difficult release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 12
[0163] Experimental Example 12 Stability Test
[0164] 1. Sources of samples and reagents: Omeprazole sodium for injection, 3 batches were self-made according to the prescription and process described in Example 1, and the batch numbers were: AZ1, AZ2, AZ3; the reference substance of omeprazole sodium was purchased from China Pharmaceuticals Institute of Biological Products.
[0165] 2. Test method:
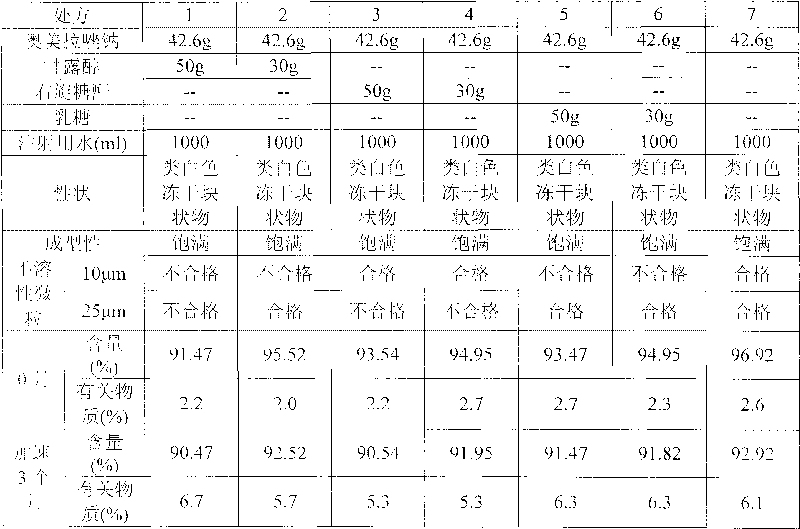
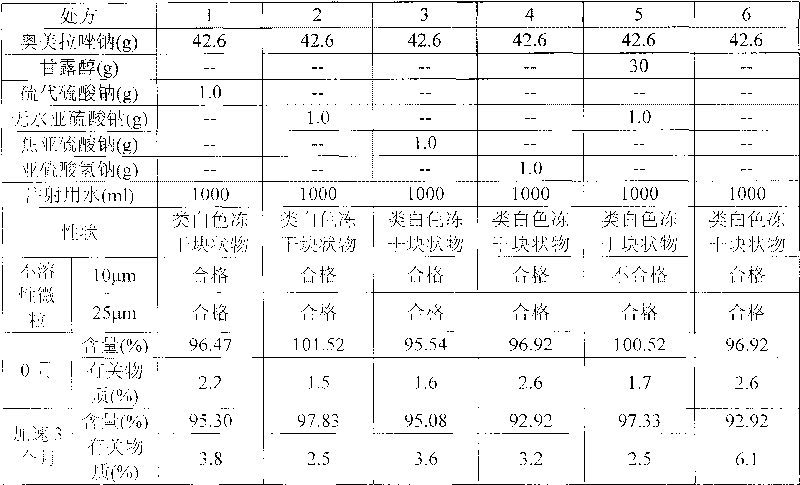
[0166] (1) Accelerated stability test: Take 3 batches of samples respectively, according to the commercially available packaging, place them for 6 months at a temperature of 40°C ± 2°C, and a relative humidity of 75% ± 5%. Samples were taken at 6 months to investigate the properties, pH value, related substances and content. The results are shown in the table below
[0167] Accelerated Stability Test Results
[0168]
[0169]
[0170] From the test results, it can be seen that the medicine of the present invention is through the accelerated test for 6 months, and the de...
Embodiment 1
[0207] Omeprazole Sodium 42.6g
[0208] Edetate Disodium 0.75g
[0209] Anhydrous sodium sulfite 1.0g
[0210] Add water for injection to: 1000ml
[0211] A total of 1000 pieces
[0212] For preparation, take about 90% of the prescription amount of water for injection at normal temperature, add disodium edetate and anhydrous sodium sulfite, and stir until completely dissolved. Add the ingredients and stir until completely dissolved. Detect the pH value of the solution, and adjust it to the range of 10.7 with NaOH solution; add 0.1% (W / V) activated carbon for needles, stir and adsorb for 20 minutes, decarbonize, sterilize and filter; add water for injection to the prescribed amount; intermediate inspection, filling;
[0213] Turn on the front box refrigeration to lower the temperature of the product to below -25°C for about 2 to 3 hours; turn on the back box refrigeration to lower the temperature of the condenser to below -35°C; pump the vacuum of the freeze-drying box to ...
Embodiment 2
[0228] Omeprazole Sodium 42.6g
[0229] Edetate Disodium 1.8g
[0230] Anhydrous sodium sulfite 0.6g
[0231] Add water for injection to: 1000ml
[0232] A total of 1000 pieces
[0233] The preparation process is the same as in Example 1, wherein the pH value of the intermediate solution is adjusted to 10.9, and the pre-freezing temperature is at -50°C.
[0234] Take the intermediate solution and finished product of the above method, and investigate the content, related substances, properties and other indicators of the sample.
[0235]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com