Thioctic acid-glucan conjugate, preparation method and usage thereof
A technology of glucan and lipoic acid, applied in the field of pharmacy, can solve the problems of unstable raw material quality and low encapsulation rate, and achieve the effect of increasing lipophilic performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] 1.0g (6.17mmol anhydrous glucose unit) dextran (molecular weight is 70,000) is dissolved in 20ml mixed solvent (formamide: dimethylformamide: dichloromethane=10:10:1 (v / v / v) ), heat at 30°C for 2 hours, add 0.64g (6.17mmol) of lipoic acid, dissolve and add 75mg (0.61mmol) of DMAP, stir to dissolve, add 0.65g (6.17mmol) of dicyclohexylcarbodiimide, and stir , 30 ℃ heat preservation reaction for 24 hours. The generated dicyclohexyl urea was removed by filtration, and the filtrate was poured into 160ml of methanol:ether (3:1, V / V) mixture, a solid precipitated, collected by filtration, washed with 50ml of ether and 50ml of acetone in turn. Dry in the presence of phosphorus pentoxide and store in a desiccator. The yield is 52.4%, the molecular weight of the obtained product is 89,000, and the substitution degree of lipoic acid on dextran is 20.1% by weight.
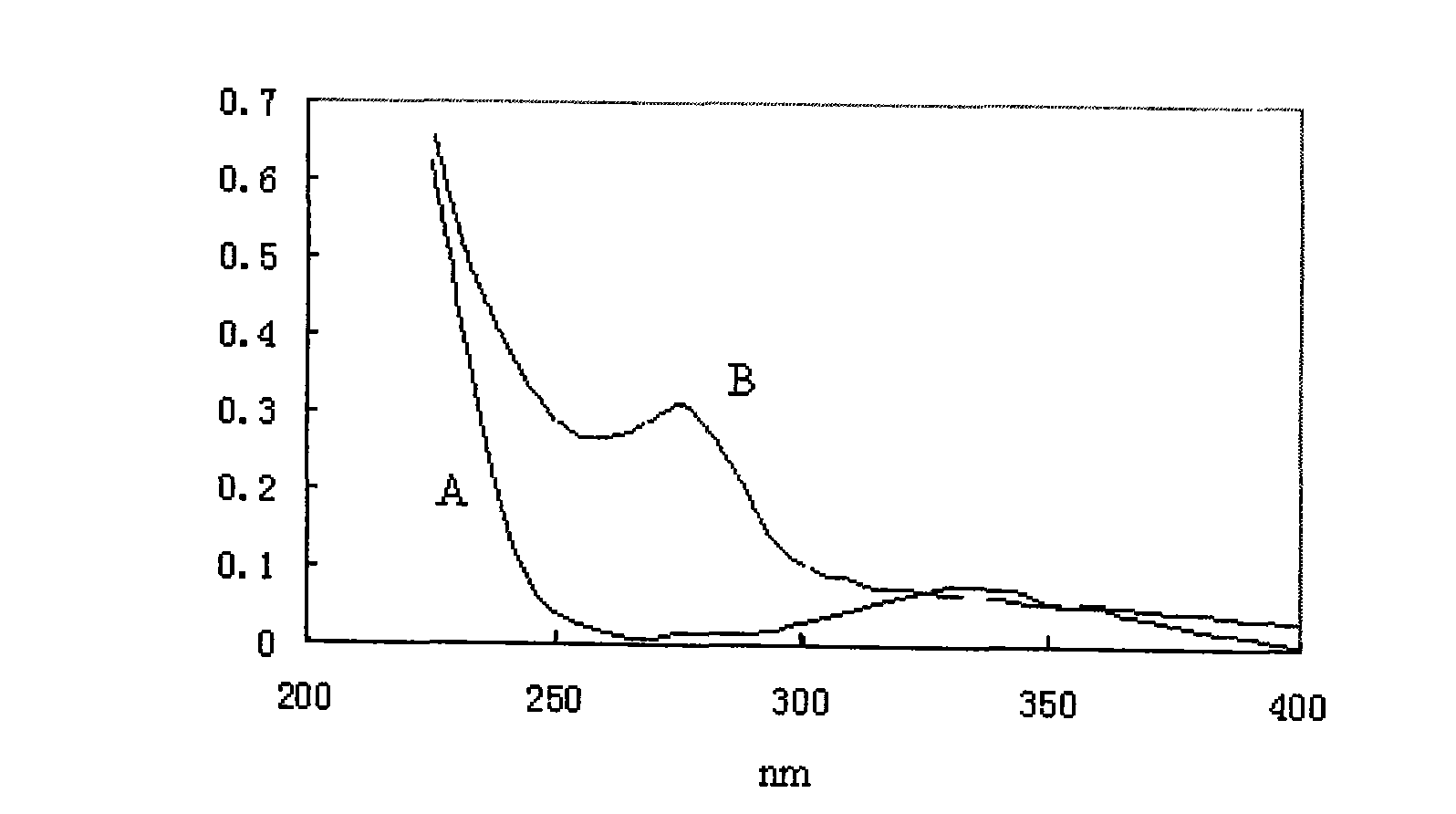
[0046] The product was confirmed by UV. at 1720cm -1 There are characteristic absorption peaks of ester bonds ne...
Embodiment 2
[0048] By the method of embodiment 1, increase the ratio of raw material lipoic acid and dextran.
[0049] 1.0g dextran (molecular weight: 40,000) was dissolved in 40ml mixed solvent (formamide: dimethylformamide: dichloromethane = 6:6:1 (v / v / v)), and kept at 40°C for 1 hour , add lipoic acid 1.28g (12.3mmol), after dissolving, add 100mg (0.82mmol) DMAP, stir to dissolve, add dicyclohexylcarbodiimide 1.30g (12.3mmol), under stirring, 40 ℃ insulation reaction for 20 hours. The generated dicyclohexylurea was removed by filtration, and the filtrate was poured into 200ml of methanol:water (1:3, V / V) mixture, a solid was precipitated, collected by filtration, washed with 50ml of methanol and 50ml of water successively. Dry in the presence of phosphorus pentoxide and store in a desiccator. The yield is 40.8%, the molecular weight of the obtained product is 74,000, and the substitution degree of lipoic acid on dextran is 45.5% by weight.
[0050] The product was confirmed by UV. a...
Embodiment 3
[0052] 1.0g (6.17mmol) of carbonyldiimidazole was dissolved in 20ml of tetrahydrofuran, at room temperature, 0.64g (6.17mmol) of lipoic acid was added, stirred for 2 hours, THF was evaporated under reduced pressure, the solid was dissolved by adding 5ml of DMSO, and then dissolved in 1.0 g (6.17 mmol of anhydrous glucose unit) dextran (70,000 molecular weight) in DMSO solution 15 ml and 0.62 g of triethylamine were stirred and reacted at 30° C. for 24 hours. The reaction solution was poured into 200ml of methanol:ether (3:1, V / V) mixture, and a solid precipitated out, which was collected by filtration and washed successively with 50ml of ether and 50ml of acetone. Dry in the presence of phosphorus pentoxide and store in a desiccator. The yield is 35.5%, the molecular weight of the obtained product is 87,000, and the substitution degree of lipoic acid on dextran is 19.5% by weight.
[0053] The product was confirmed by UV. at 1720cm -1 There are characteristic absorption pea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com