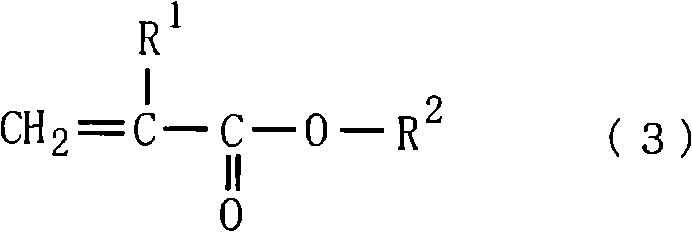Photo- and/or thermo-curable copolymer, curable resin compositions, and cured articles
A thermosetting, copolymer technology, applied in the field of cured products, can solve the problems of inability to obtain curable resin and increased viscosity of resin solution, and achieve the effects of excellent storage stability, good curing characteristics, and excellent solvent resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0144] Hereinafter, the present invention will be described in more detail based on examples, but the present invention is not limited by these examples. The weight-average molecular weight and degree of dispersion of the copolymer were measured by GPC (gel permeation chromatography; polystyrene conversion).
[0145] (Synthesis of Copolymer)
preparation example 1
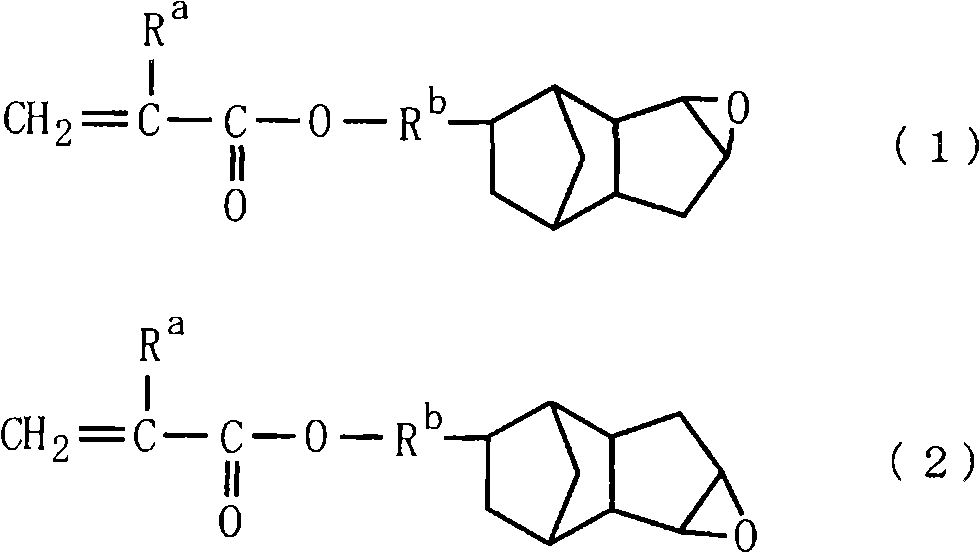
[0147] Add 130 g of methoxybutyl acetate and 110 g of methoxybutanol to a separable flask with an internal volume of 1 liter of a stirrer, a thermometer, a reflux condenser, a dropping funnel, and a nitrogen introduction pipe, and heat up to 80° C. Add 85g of methacrylic acid, 265g of 3,4-epoxytricyclo [5.2.1.0] dropwise over a period of 5 hours. 2,6 ]decane-9-yl acrylate and 3,4-epoxytricyclo[5.2.1.0 2,6 ] a mixture of decan-8-yl acrylate [50:50 (molar ratio)] [the compound represented by the above-mentioned formula (9), Ra'=H], and 30 g of azobisdimethylvaleronitrile dissolved in 380 g of acetic acid The mixed solution obtained in methoxybutyl ester was further aged for 3 hours to obtain a copolymer (P-1) having a carboxyl group. The reaction was carried out under nitrogen flow. The obtained copolymer had a solid content of 35.4% by weight, an acid value of 158KOH mg / g, a weight average molecular weight (Mw) of 11200, and a degree of dispersion of 1.96.
preparation example 2
[0149] Add 130 g of methoxybutyl acetate and 110 g of methoxybutanol to a separable flask with an internal volume of 1 liter of a stirrer, a thermometer, a reflux condenser, a dropping funnel, and a nitrogen introduction pipe, and heat up to 80° C. 45 g of methacrylic acid, 131 g of ω-carboxy-polycaprolactone monoacrylate (Aronics (Aronics) M5300: manufactured by Toagosei Co., Ltd.), 173 g of 3,4-epoxytricyclic [5.2.1.0 2,6 ]decane-9-yl acrylate and 3,4-epoxytricyclo[5.2.1.0 2,6 ] a mixture of decan-8-yl acrylate [50:50 (molar ratio)] [the compound represented by the above-mentioned formula (9), Ra'=H], and 30 g of azobisdimethylvaleronitrile dissolved in 380 g of acetic acid The mixed solution obtained in methoxybutyl ester was further aged for 3 hours to obtain a copolymer (P-2) having a carboxyl group. The reaction was carried out under nitrogen flow. The obtained copolymer had a solid content of 35.5% by weight, an acid value of 154KOH mg / g, a weight average molecular w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| acid value | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| weight-average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com