Hepatitis C virus variation detection protein chip, preparation method and application thereof
A hepatitis C virus and protein chip technology, applied in the field of hepatitis virus detection protein chip, can solve the problems of lack of clinical significance, expensive laser confocal scanner, and no visible protein chip for detection of hepatitis C virus, etc. good sex effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 HCV mutation detection protein chip reaction matrix design and protein chip preparation
[0030]1. Materials: anion exchange column Q-Sepharose FF, gel filtration column Sephardex G-50 were purchased from Pharmacia Biotech; urea and sucrose were purchased from Beijing Chemical Reagent Company; lysozyme and BSA were purchased from Promega; aminosilane The chemical glass slides were purchased from Beijing Boao Biochip Co., Ltd.; the ultrasonic crushing instrument was purchased from Ningbo Xinzhi Technology Co., Ltd.
[0031] 2. Method results:
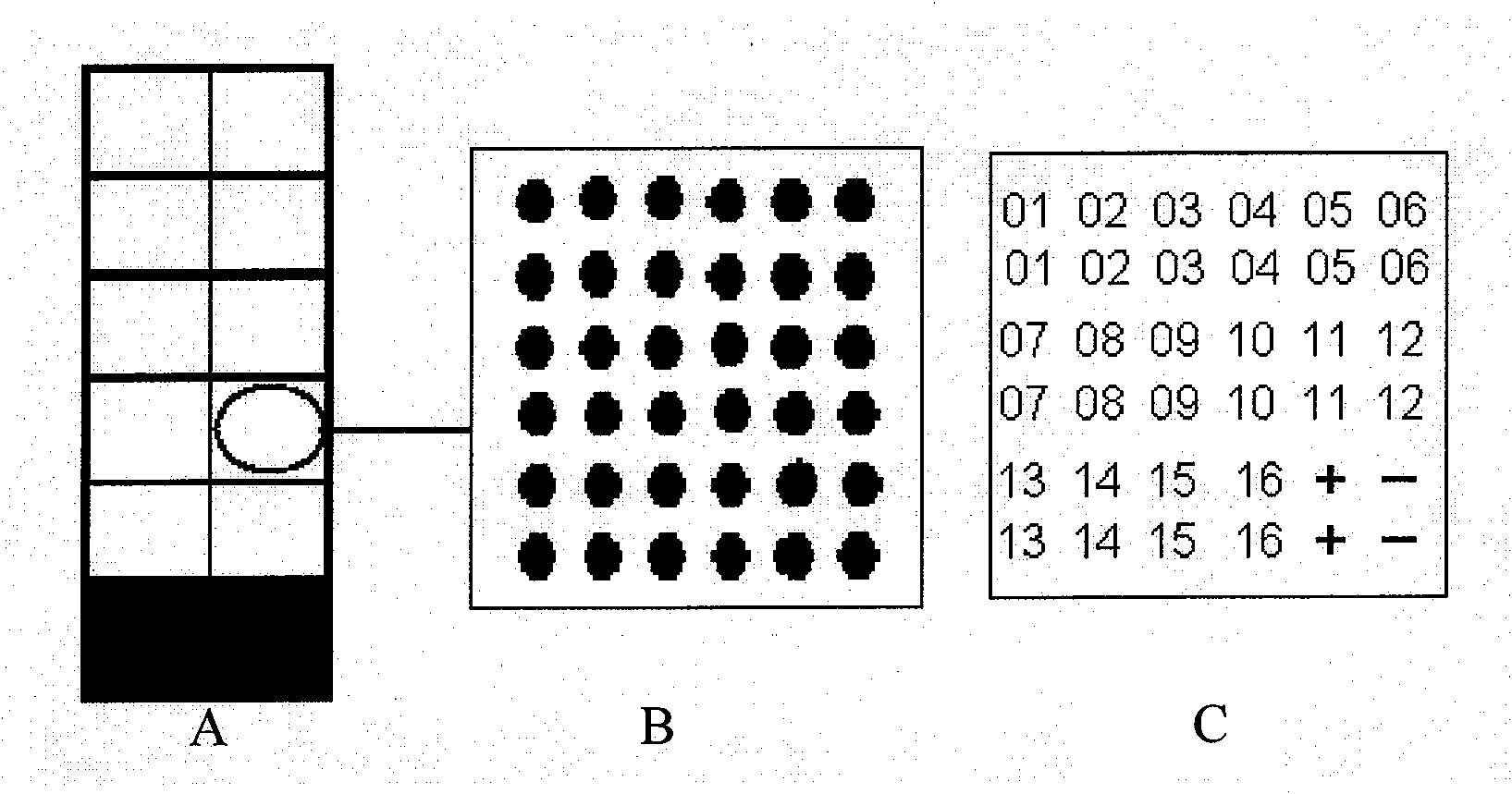
[0032] 1. We designed a 6×6 reaction matrix consisting of 16 multi-target HVR1 antigens. Each antigen was replicated vertically twice, and a positive IgG control and an IL1 negative control were set up (attached figure 1 ).
[0033] 2. Cultivation of expression strains: Take 20 μl of expression strains stored at -70°C (preserved in this room. The first hypervariable region antigen of hepatitis C virus and its use in th...
Embodiment 2
[0040] Example 2 HCV mutation detection protein chip reaction and visualization of results
[0041] 1. Materials: Colloidal gold-labeled protein A: purchased from Beijing Zhongshan Biotechnology Company; hydroquinone sodium citrate, citric acid, and silver nitrate were purchased from Beijing Chemical Reagent Company;
[0042] 2. Method results:
[0043] Take the chips out of the refrigerator and rewarm for 20 minutes, wash the chips with pure water, and suck out excess water. The serum was diluted 10 times with the chip diluent, dropped onto the chip reaction area, and kept at room temperature for 1 hour. Wash the slices 5 times with washing solution, wash the slices with pure water and suck out excess water. Add colloidal gold-labeled protein A (diluted with PBS 1:100), react at room temperature for 10 minutes, wash the slices with washing solution 5 times, wash the slices with pure water, suck out excess water, add silver chromogenic solution (solution A: terephthalate Di...
Embodiment 3
[0044] Example 3 Clinical significance of visualized HCV mutation detection protein chip
[0045] 1. Materials: 28 serum samples of asymptomatic HCV infection were provided by Beijing Red Cross Blood Center; 41 serum samples of patients with chronic hepatitis C and 37 serum samples of patients with liver cirrhosis were provided by People's Hospital Affiliated to Peking University; serum samples before and after interferon treatment for hepatitis C were provided by Provided by the Second Affiliated Hospital of Chongqing Medical University;
[0046] 2. Method results:
[0047] 1. Visualize the relationship between HCV mutation detection protein chip and disease chronicity process
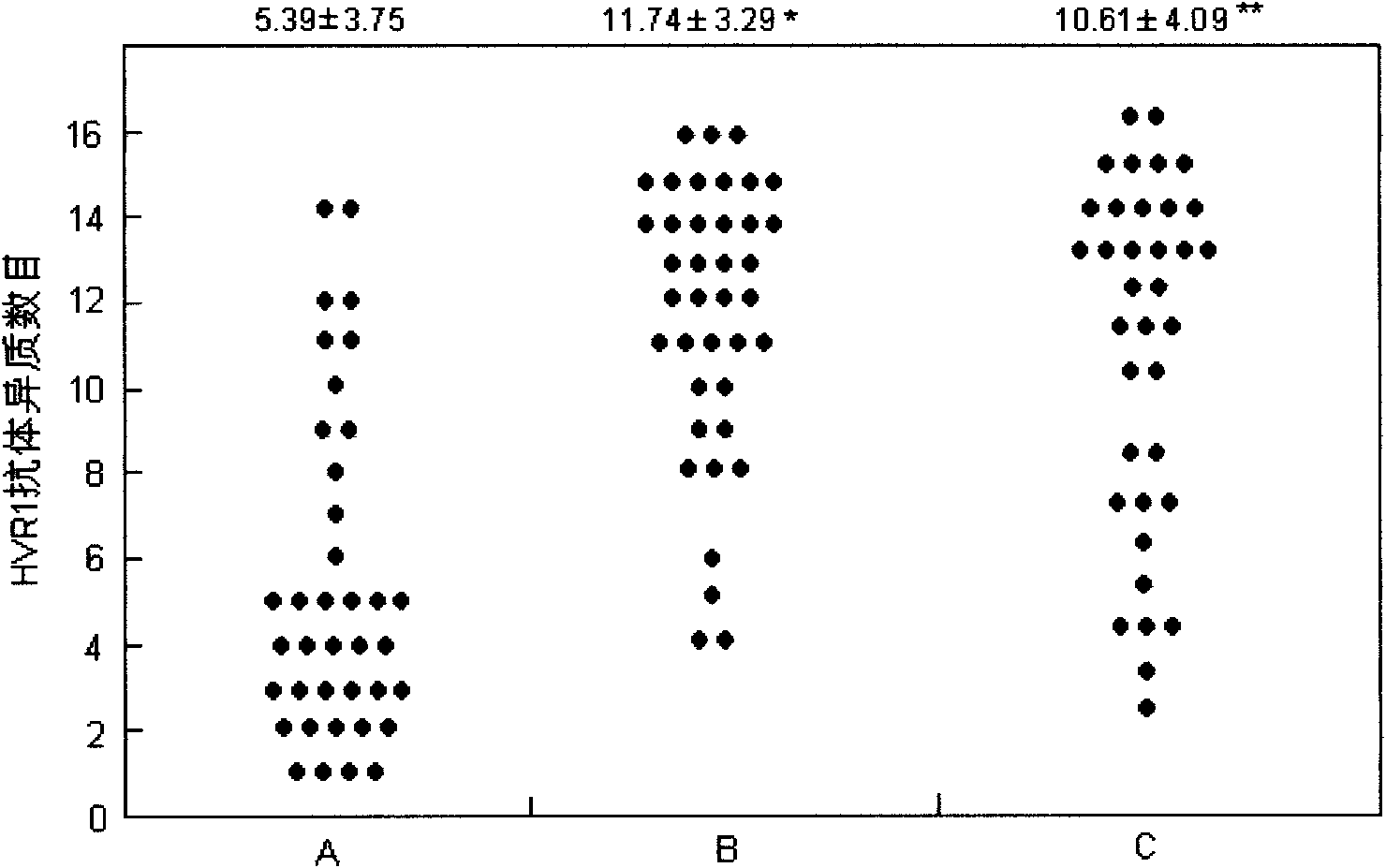
[0048] The heterogeneity of HVR1 antibody in 28 sera of asymptomatic HCV patients, 41 sera of chronic patients and 37 sera of patients with liver cirrhosis was detected by visual HCV mutation detection protein chip. The result is as image 3 Shown: The number of positive multi-target HVR1 antibodie...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com