Stable controlled-release rasagiline transdermal patch and preparation method thereof
A transdermal patch, stable technology, applied in the direction of medical preparations with non-active ingredients, medical preparations containing active ingredients, sheet-like delivery, etc., can solve the problems of unfavorable long-term storage and poor drug stability, and achieve Easy to prepare and long-lasting effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Example 1: A single-layer polyacrylate matrix patch containing rasagiline mesylate, using lauryl acid as a skin penetration promoting agent
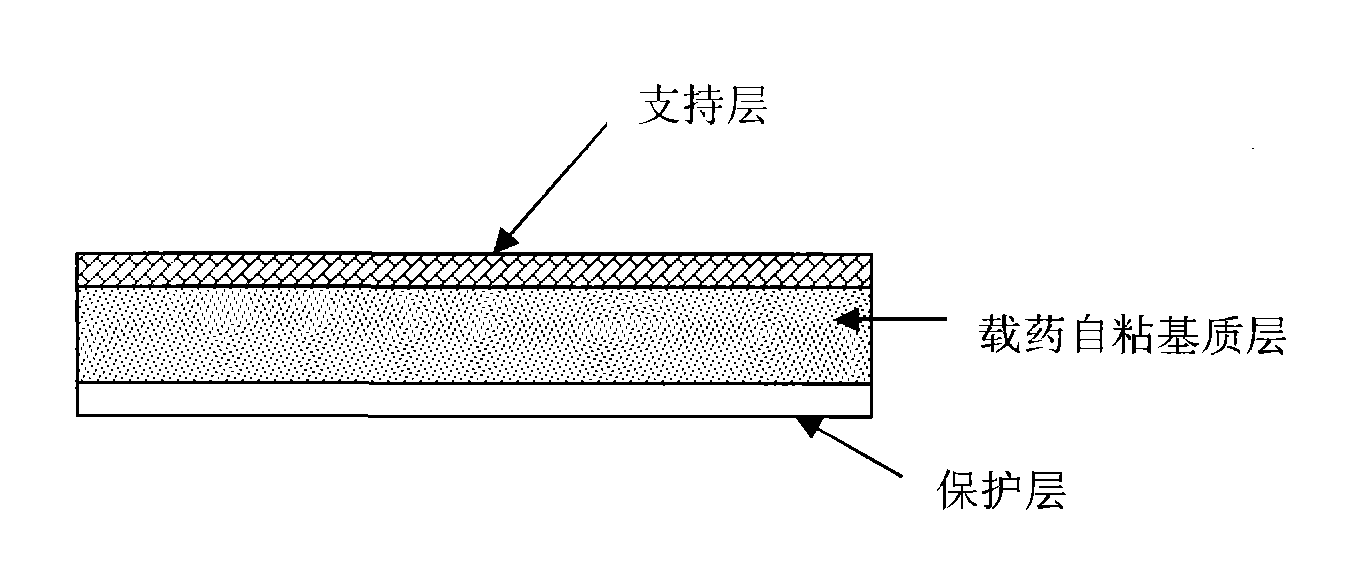
[0067] 0.25 g of rasagiline mesylate was dissolved in 1 g of propylene glycol, and added to 20 g of polyacrylate glue (National Starch, 387-2287). Add 0.3 g of oleic acid, and after stirring evenly, use an automatic coating machine (Shanghai Kaikai Company, TB-04 type) to coat the mixed glue solution on the siliconized paper with a coating thickness of 0.6 mm. After drying at 60°C for 5 minutes, cover the support layer and transfer it, and then die-cut or cut the patch into the final sheet. The measured rasagiline content was 220ug / cm 2 , The measured pH is 4.4. The cross-sectional schematic diagram of the prepared patch is shown in figure 1 .
[0068] The penetration of the prepared patch was studied using a Franz cell and the depilated abdominal skin of two-month-old rats. Use high-performance liquid chromatography to measu...
Embodiment 2
[0071] Example 2: A single-layer silicone matrix patch containing rasagiline, using laurazozone as a skin penetration promoting agent
[0072] Take 0.25 gram of rasagiline free base and dissolve it in 1 gram of propylene glycol, and then add it to 20 grams of silicone gel (3M Company, USA, PSA7-4302 type). Add 0.3 g of laurocaprazol, and after stirring evenly, take a small amount of glue as a sample (for stability research) and measure the pH value as 7.7. Prepare according to the above prescription and process, and add 0.15 g of methanesulfonic acid and stir evenly to obtain a glue solution. Take a small amount of the glue solution as a sample to measure (for stability research) and get a pH value of 4.0. Use an automatic coating machine (Shanghai Kaikai Company, TB-04 type) to coat the mixed glue solution on the siliconized paper with a coating thickness of 0.6 mm. After drying at 60°C for 5 minutes, cover the support layer and transfer it, and then die-cut or cut the patch...
Embodiment 3
[0077] Example 3: Single-layer polyvinylpyrrolidone-polyethylene glycol composite matrix patch containing rasagiline, using laurazozone as skin penetration promoting agent
[0078] Take 24 grams of polyvinylpyrrolidone PVP-K90D (ISP Technologies, INC) and sprinkle it into 120 grams of absolute ethanol, stir to make it swell completely, add 16 grams of polyethylene glycol 400 (Nanjing Weier Chemical Industry) into it, stir and mix Evenly, a blank glue is prepared. Take 15 grams of the prepared glue solution, add 1.0 grams of rasagiline free base, stir and ultrasonically dissolve it. Add 0.3 gram of azoxazone, and after stirring evenly, the measured pH value is 7.4. Prepare according to above-mentioned prescription, process, and add 0.6 gram of methanesulfonic acid, record pH value to be 4.7. Use an automatic coating machine (Shanghai Kaikai Company, TB-04 type) to coat the mixed glue solution on the siliconized paper with a coating thickness of 0.4 mm. After drying at 60°C f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com