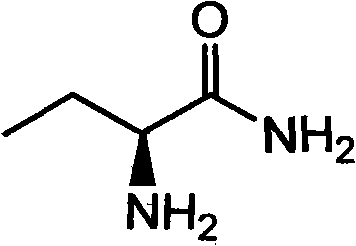
Production method of S-2-aminobutanamide
A technology of aminobutyramide and production method is applied in the preparation of carboxylic acid amides, chemical instruments and methods, preparation of optical isomers of carboxylic acid amides, etc., and achieves the effects of less investment in equipment, high safety, and reduced production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
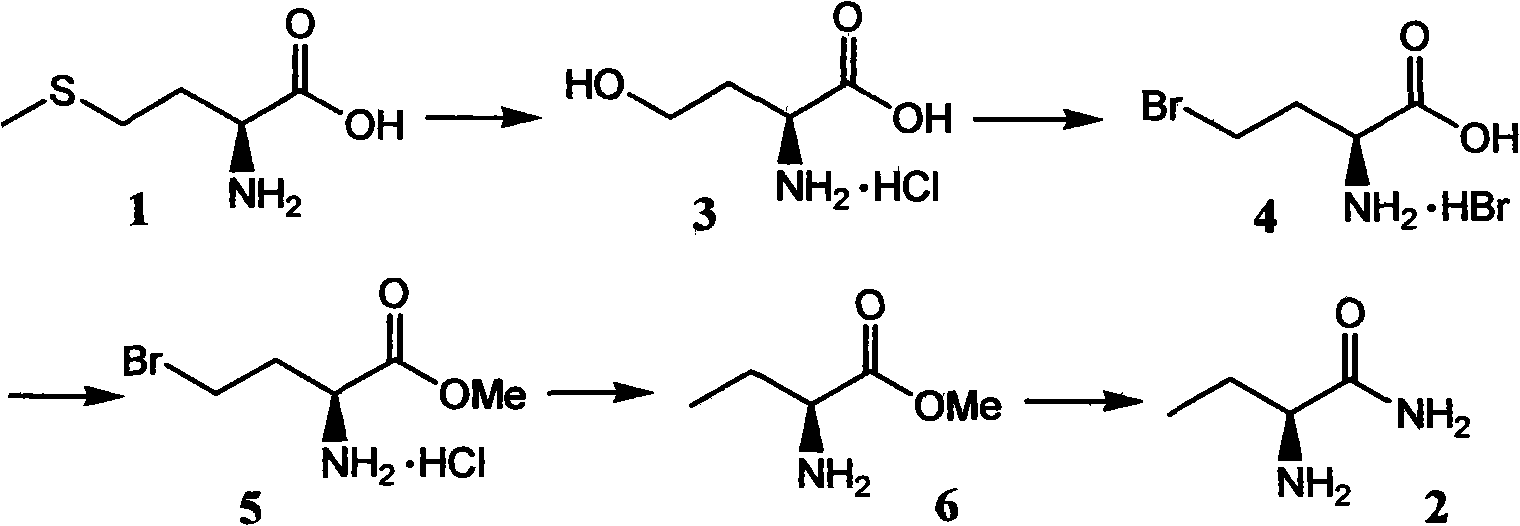
[0030] 14.9 grams (100 mmoles) of L-(+)-methionine were dissolved in a mixed solvent of 250 milliliters of water and 70 milliliters of ethanol, the temperature was raised to 30-60° C., and 27.3 grams of ethyl 2-chloropropionate ( 200 mmol), after the addition, stir and react at the reaction temperature for 8 to 24 hours, then heat and reflux for 4 to 8 hours, cool to room temperature, add 100 ml of ethyl acetate to the system to extract once, wash twice , remove the solvent in the aqueous phase under reduced pressure, and filter to obtain a solid crude product, which is recrystallized in 350 mL of water-ketone-alcohol solvent to obtain pure white crystalline product S-2-amino-4-hydroxyl Butyrate hydrochloride 13.3 g (yield 85%), melting point 203 ° C, specific rotation -8.5 ° C (c = 2, H 2 O 22°C).
[0031] In an autoclave, dissolve S-2-amino-4-hydroxybutyrate hydrochloride (4.68 g, 30 mmol) in 30 ml of 33% HBr / AcOH solution, heat to 65-80 °C, and seal the reaction for 6 hou...
Embodiment 2
[0036]Dissolve 8.94 grams (60 mmol) of L-(+)-methionine in a mixed solvent of 200 milliliters of water and 30 milliliters of ethanol, raise the temperature to 30-60 °C, and add 6.5 grams of methyl dichloroacetate (60 milliliters) dropwise thereto. mol), after the addition, stir the reaction at the reaction temperature for 8 to 24 hours, then heat and reflux for 4 to 8 hours, cool to room temperature, add 60 milliliters of ethyl acetate to the system to extract once, wash twice, reduce The solvent in the aqueous phase was removed under pressure, filtered to obtain a solid crude product, and the obtained solid crude product was recrystallized in 200 mL of water-ketone-alcohol solvent to obtain a pure white crystalline product S-2-amino-4-hydroxybutyric acid Hydrochloride 7.58 grams, yield 81%, melting point 203 ° C, specific rotation -8.5 ° C (c = 2, H 2 O 22°C).
[0037] In an autoclave, dissolve S-2-amino-4-hydroxybutyrate hydrochloride (9.36 g, 60 mmol) in 50 ml of 30% HBr / A...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com