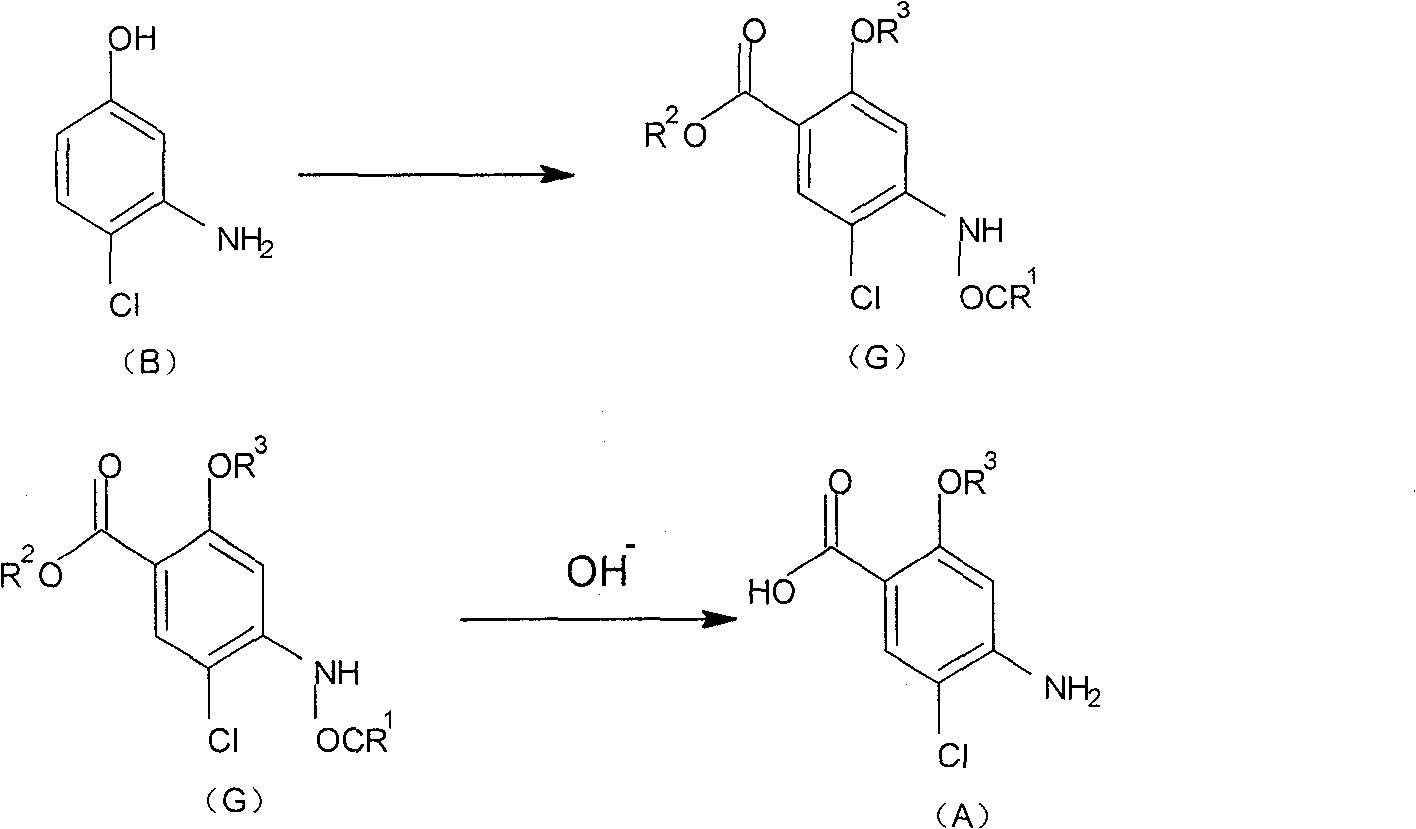
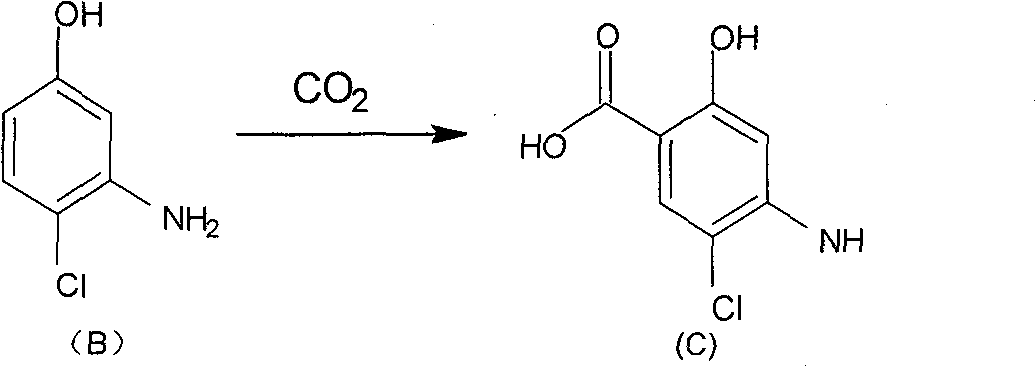
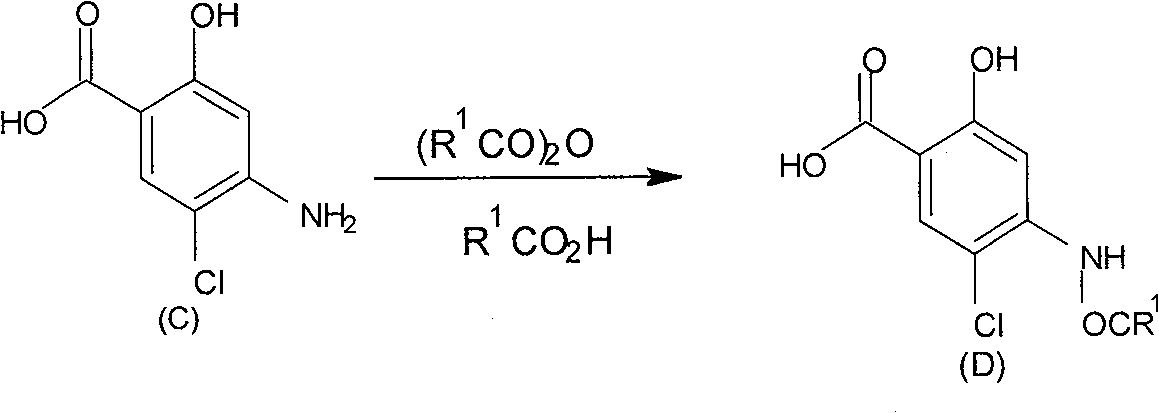Method for preparing 2-alkoxy-4-amino-5-chlorobenzoic acid
A technology of chlorobenzoic acid and alkoxy, which is applied in the field of preparation of 2-alkoxy-4-amino-5-chlorobenzoic acid, which can solve the difficulties in the treatment of nitrogen-containing wastewater, the inability to recycle DMF, and the existence of safety hazards, etc. problems, to avoid serious problems in production equipment, avoid safety and environmental hazards, and reduce raw material costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1: Preparation of 4-amino-5-chloro-2-hydroxyl-benzoic acid (C)
[0021]
[0022] Method (1), add 43 grams of compound B, 41.5 grams of anhydrous potassium carbonate, and 300 milliliters of xylene into a 1 L reaction flask, and heat to reflux for 3 hours. Transfer the reaction solution into a 1L autoclave, replace the air in the autoclave twice with nitrogen, and slowly introduce carbon dioxide gas to the autoclave pressure of 7 kg / cm 2 , heating up to 120°C. In the kettle temperature 120-140 ℃, kettle pressure 10-15 kg / cm 2 React for 6-9 hours. The reaction kettle was cooled to room temperature, 500 ml of water was added, the water layer was separated, and the toluene layer was extracted twice with water. Combine the aqueous layers, adjust the pH to less than 2 with concentrated hydrochloric acid, and cool to precipitate crystals. Crude product was obtained by filtration. Recrystallized with acetone to obtain 46.9 g of compound C: melting point 188-18...
Embodiment 2
[0025] Embodiment 2: Preparation of 4-acetylamino-5-chloro-2-hydroxyl-benzoic acid (D)
[0026] Method A:
[0027]
[0028] Add 19 g of compound C, 30 ml of glacial acetic acid, and 20 ml of acetic anhydride into a 100 ml three-necked flask, stir at room temperature for 3 hours, evaporate about 30 ml of solvent under reduced pressure, cool the residue to room temperature, and drop 200 ml of water, precipitated, cooled, filtered, and washed with water to obtain a crude product. The crude product was recrystallized from ethanol / water to obtain 1.2 g of compound D2. The melting point is 240-242.5°C. The yield is 90.8%.
[0029] Method B:
[0030]
[0031] With reference to the method of above-mentioned method A, product B' is prepared from B with a yield of 92.2%.
[0032] With reference to the method (1) of embodiment 1, the yield 72.4% of product D is prepared by B '.
[0033] With reference to the method (2) of Example 1, the yield of Compound D prepared from Compou...
Embodiment 3
[0035] Embodiment 3: Preparation of 4-acetylamino-5-chloro-2 ethoxy-ethyl benzoate (G')
[0036]
[0037] Put 23 grams of compound D, 200 milliliters of acetone, 30 grams of anhydrous potassium carbonate, and 50 milliliters of diethyl sulfate into the reaction flask, heat and reflux for 5 hours, distill the solvent, cool the residue, add 200 milliliters of water, and adjust the pH to 8 with dilute hydrochloric acid , filtered, and the cake was washed with 95% ethanol and dried to obtain 26.3 g of light yellow solid, yield 92.0%, Mp147-149°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com