Preparation method of multistage drug release carrier compounded by gelatin microspheres and calcium phosphate cement
A calcium phosphate bone cement and gelatin technology, applied in prosthesis, medical science, bone implants, etc., can solve the problem of not being able to meet the timing and quantitative release of various drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Step 1: Preparation of gelatin microspheres
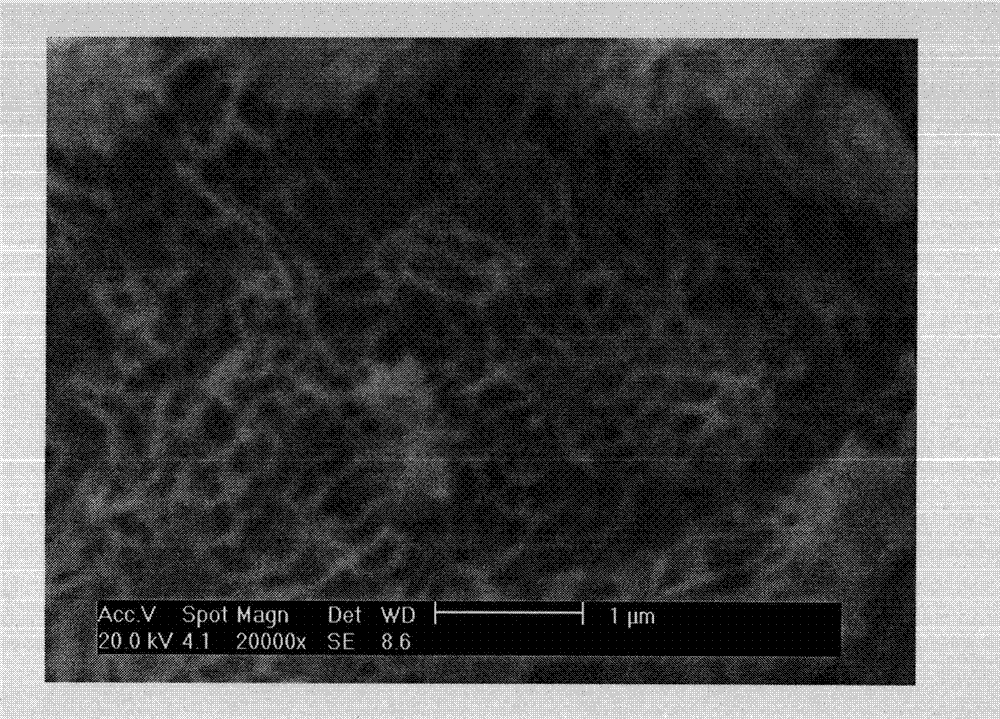
[0020]Dissolve 3g of gelatin in 30mL of deionized water at a temperature of 40°C to prepare a gelatin solution with a concentration of 0.10g / mL; then add the gelatin solution into 125mL of vegetable oil with continuous stirring (stirring speed 700rpm) through a porous nozzle. Keep at 15°C, stir for 10 minutes to form an emulsion, quickly change to an ice bath while keeping the stirring speed constant, keep the system temperature below 4°C, continue stirring for 15 minutes, slowly add 5mL of glutaraldehyde with a concentration of 25% dropwise Solution, continue to stir for 0.5h, after filtering, soak the microspheres in the glycine solution with a concentration of 1mol / L for 30min, remove unreacted glutaraldehyde, and wash the prepared microspheres with acetone and ethanol for 3 times, and dry naturally The gelatin microspheres (such as figure 1 shown), the microspheres were sieved and classified to obtain gelatin microspher...
Embodiment 2
[0026] Step 1: Preparation of gelatin microspheres
[0027] Dissolve 9g of gelatin in 30mL of deionized water at a temperature of 40°C to prepare a gelatin solution with a concentration of 0.3g / mL; then add the gelatin solution dropwise into 125mL of vegetable oil with continuous stirring (stirring speed 500rpm) with a syringe, and the oil Keep the temperature at 38°C, stir for 30 minutes to form an emulsion, quickly change to ice bath under the condition of keeping the stirring speed constant, keep the system temperature below 4°C, continue stirring for 30 minutes, slowly add 10mL of 25% pentadiol dropwise Aldehyde solution, continue to stir for 0.5h, after filtration, soak the microspheres in 1mol / L glycine solution for 30min, remove unreacted glutaraldehyde, wash the prepared microspheres with acetone and ethanol three times in sequence, and dry naturally to obtain The gelatin microspheres with a particle size ranging from 100 to 250 μm are sieved and classified to obtain g...
Embodiment 3
[0033] Step 1: Preparation of gelatin microspheres
[0034] Dissolve 9g of gelatin in 30mL of deionized water at a temperature of 40°C to prepare a gelatin solution with a concentration of 0.3g / mL; then add the gelatin solution dropwise with a syringe to 125mL of vegetable oil with continuous stirring (stirring speed 300rpm). Keep the temperature at 50°C, stir for 30 minutes to form an emulsion, quickly change to ice bath under the condition of keeping the stirring speed constant, keep the system temperature below 4°C, continue stirring for 60 minutes, slowly add 10mL of 50% pentadiol dropwise aldehyde solution, continue to stir for 1 h, after filtration, soak the microspheres in 1mol / L glycine solution for 30 min to remove unreacted glutaraldehyde, wash the prepared microspheres with acetone and ethanol three times in sequence, and dry naturally to obtain particles Gelatin microspheres with diameters ranging from 75 to 150 μm were sieved and classified to obtain gelatin micro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com