Method for synthesizing hydrogel
A technology of hydrogel and methacrylic acid, applied in the field of synthetic hydrogel, can solve the problems of slow stimulus response, limited application, lack of regularity, etc., and achieves the advantages of being beneficial to industrial production, good biocompatibility, and simple preparation method. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Synthesis of PPEGMA Hydrogel Using Br-PEG-Br as Initiator
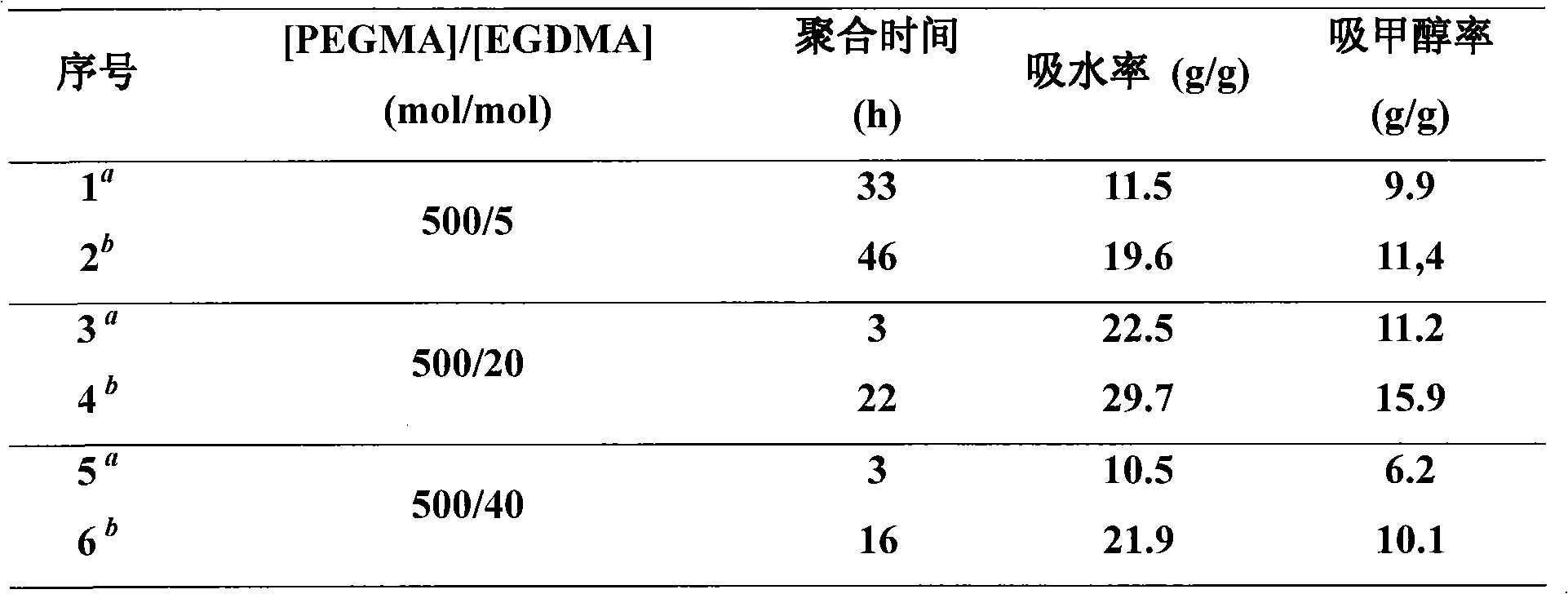
[0034] According to the ratio n(PEGMA):n(Br-PEG-Br):n(EGDMA):n(FeCl 3 .6H 2 O):n(TDA-1):n(VC)=500:1:5~40:3:9:5, add FeCl 3 .6H 2 O, TDA-1, PEGMA (2mL), EGDMA, Br-PEG-Br and VC in 10mL ampoules, sealed directly under air atmosphere (bulk polymerization). If solution polymerization is used, add 2 mL of deionized water after adding all the above chemical reagents and seal the tube directly under air atmosphere. The sealed ampoule was placed in an oil bath at a constant temperature (90° C.) for a predetermined time to react (3˜46 h). After the reaction is over, take out the sealed tube, immediately cool it with cold water, open the sealed tube, soak and filter with an appropriate amount of deionized water, and freeze-dry to obtain a xerogel.
[0035] Using macromolecular bifunctional initiator (Br-PEG-Br), by changing the amount of crosslinking agent (EGDMA), the liquid absorption rate of the obtained polymer ...
Embodiment 2
[0041] Synthesis of PPEGMA Hydrogel Prepared with DMDBH as Initiator
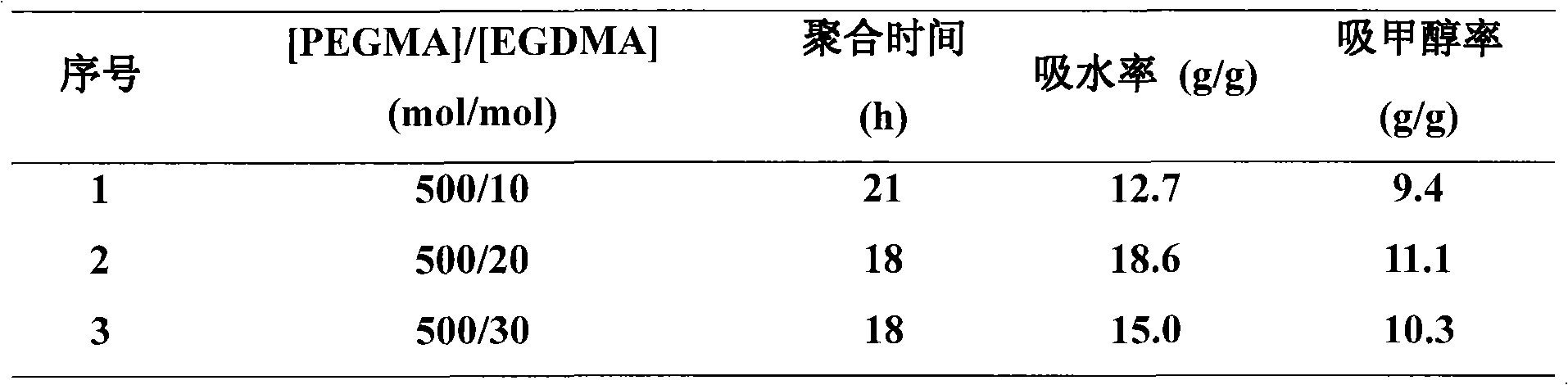
[0042] According to the ratio n(PEGMA):n(DMDBH):n(EGDMA):n(FeCl 3 .6H 2 O):n(TDA-1):n(VC)=500:1:10~30:3:9:3, add FeCl in sequence 3 .6H 2 O, TDA-1, PEGMA (2mL), EGDMA, DMDBH, VC and deionized water (2mL) were directly capped in a 10mL ampoule under air atmosphere. The sealed ampoule was placed in an oil bath at a constant temperature (90° C.) for a predetermined time to react (18-21 h). After the reaction is over, take out the sealed tube, immediately cool it with cold water, open the sealed tube, soak and filter with an appropriate amount of deionized water, and freeze-dry to obtain a xerogel.
[0043] Using a small molecule initiator (DMDBH), by changing the amount of crosslinking agent (EGDMA), the data of the liquid absorption rate of the obtained polymer hydrogel (with deionized water and methanol as the solvent to be absorbed) are shown in Table 2 Show. It can be seen from the data in the table ...
Embodiment 3
[0048] Synthesis of PDMAEMA Hydrogel
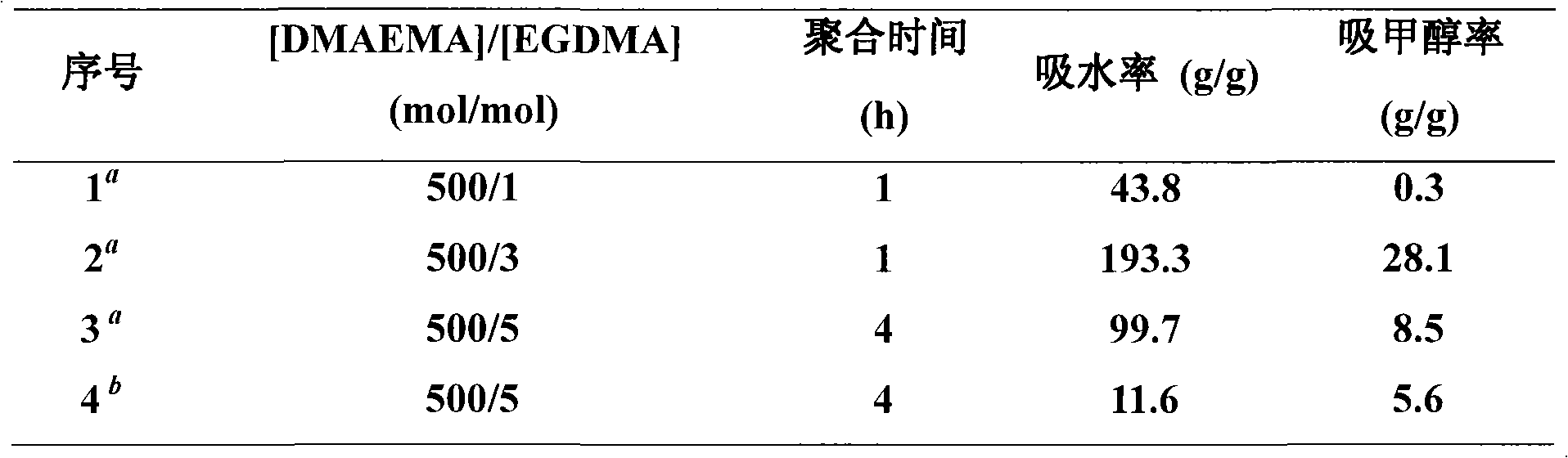
[0049] According to the ratio n(DMAEMA):n(DMDBH or Br-PEG-Br):n(EGDMA):n(FeCl 3 .6H 2 O):n(TDA-1):n(VC)=500:1:1~5:3:9:1~5, add FeCl in sequence 3 .6H 2 O, TDA-1, PEGMA (2mL), EGDMA, DMDBH or Br-PEG-Br and VC in 10mL ampoules were directly sealed under air atmosphere. The sealed ampoule was placed in an oil bath at a constant temperature (90° C.) for a predetermined time to react (1˜4 h). After the reaction is finished, take out the sealed tube, immediately cool it with cold water, open the sealed tube, soak and filter with an appropriate amount of deionized water, freeze-dry to obtain a xerogel.
[0050] Adopting small molecule bifunctional initiator (DMDBH), by changing the consumption of cross-linking agent (EGDMA), the data of the liquid absorption rate of the obtained polymer hydrogel (respectively with deionized water and methanol as the solvent to be absorbed) are shown in the table 3 (number 1, 2, 3). It can be seen from the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com