Perylene imide bridge type dimethylate-ethylenediamine-Beta-cyclodextrin derivate, preparation and applications thereof
A technology of peryleneimide bridge and ethylenediamine group, which is applied in the cross-research fields of chemistry, materials and nanotechnology, and can solve the problems of unreported research on peryleneimide derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Synthesis of peryleneimide-bridged bispermethylated-ethylenediamine-β-cyclodextrin derivatives
[0041] The synthetic route of peryleneimide bridged double permethylated-ethylenediamine-β-cyclodextrin derivatives is as follows Figure 20 shown.
[0042] at room temperature will
[0043] 6-deoxy-6-ethylenediamino-permethylated-β-cyclodextrin (0.98g, 0.67mmol),
[0044] Perylenetetracarboxylic anhydride (130mg, 0.33mmol) and zinc acetate (0.073g, 0.33mmol)
[0045] Added to 200 ml of pyridine solution;
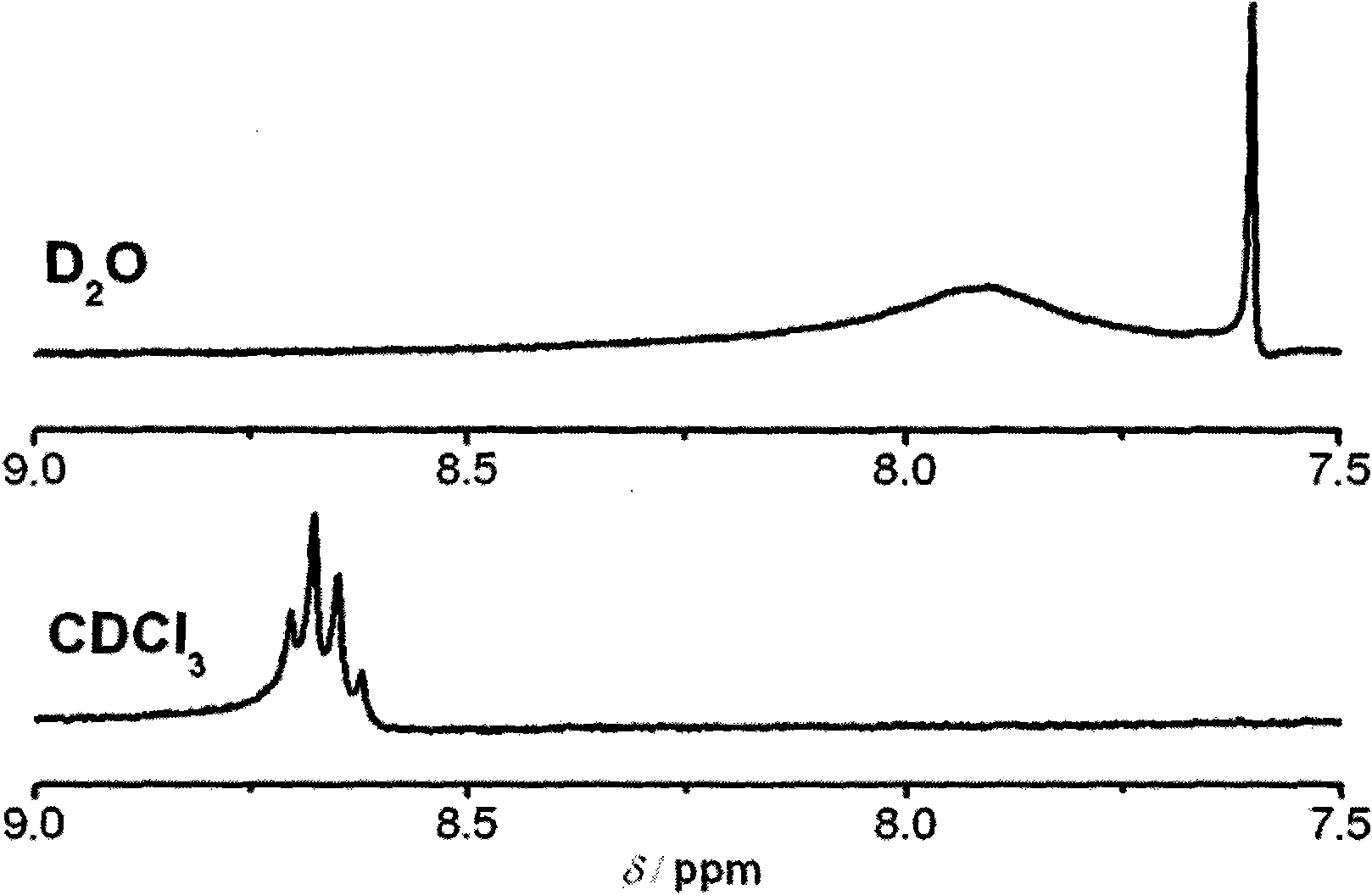
[0046] The reaction system is heated to 80-120°C and kept at 80-120°C for 48-72 hours. The reaction was stopped, cooled to room temperature, and pyridine was evaporated under reduced pressure to obtain a red solid. Dissolve the red solid in chloroform, add a small amount of water to wash away zinc acetate, dry the chloroform phase with anhydrous sodium sulfate for 12 hours, filter to remove sodium sulfate, concentrate the chloroform phase, pass through a silica gel c...
Embodiment 2
[0049] Aggregation Behavior of PTCDI 2 in Different Solvents
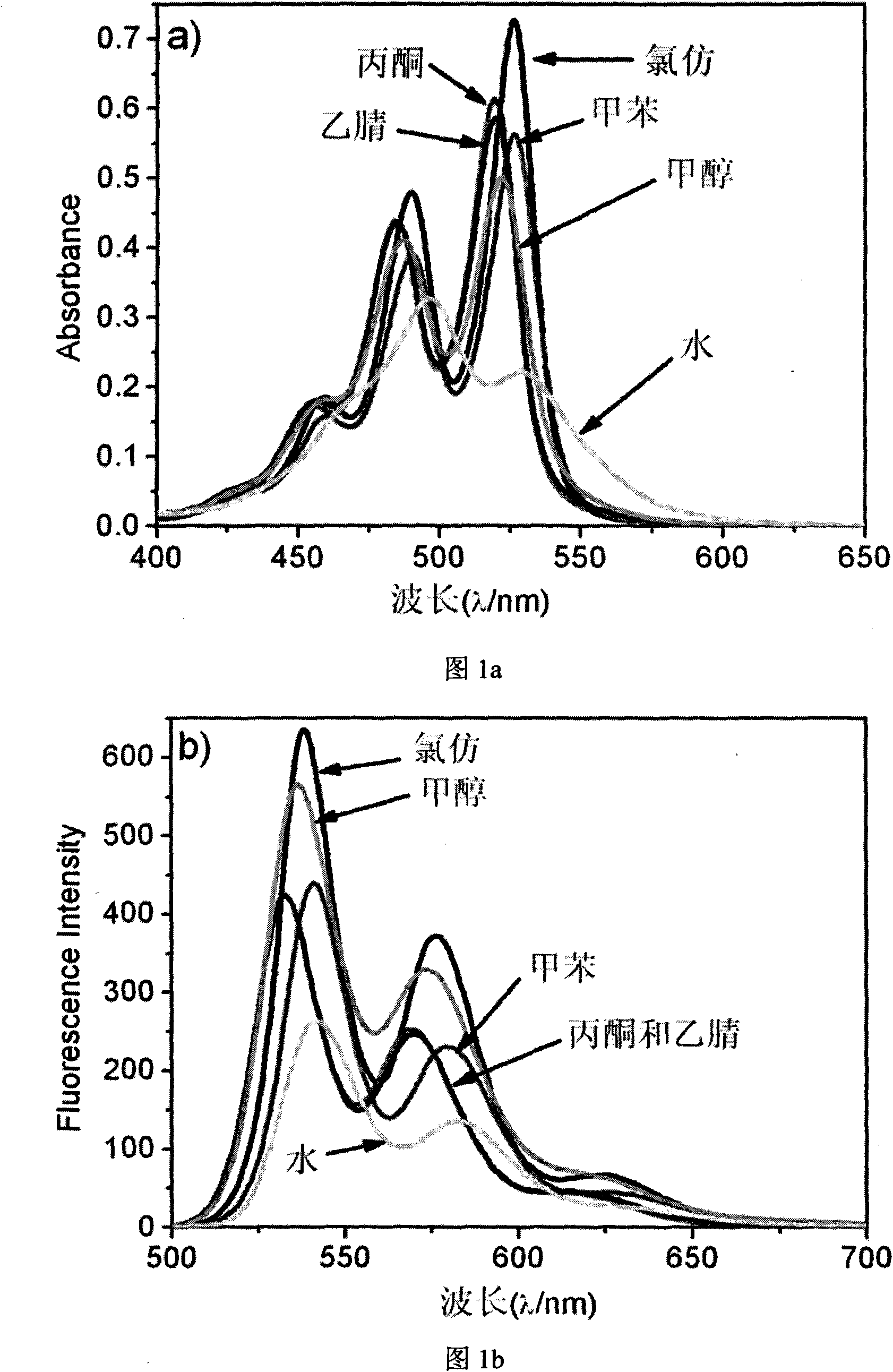
[0050] In organic solvents such as chloroform, toluene, acetone, acetonitrile, etc., PTCDI 2 presents the characteristic of ultraviolet absorption spectrum of a typical monomer, and its A 0-0 / A 0-1 Respectively 1.52 (chloroform), 1.44 (toluene), 1.40 (acetone), 1.35 (acetonitrile), and in the methanol solution, it is the spectral characteristic of monomer to dimer transformation, its A 0-0 / A 0-1 is 1.22, the maximum absorption peak is around 520nm for the 0-0 peak, and around 490nm for the 0-1 peak. However, in aqueous solution, the absorption intensity of the 0-0 peak decreases significantly, and the 0-1 peak becomes the strongest absorption peak, red shifted to 497nm, and its A 0-0 / A 0-1 reduced to 0.68, which is the characteristic absorption spectrum of the aggregate state, such as figure 1 a, At the same time, the fluorescence intensity of PTCDI 2 in aqueous solution is obviously weaker than that in org...
Embodiment 3
[0052] Spectral study of PTCDI 2 before and after protonation
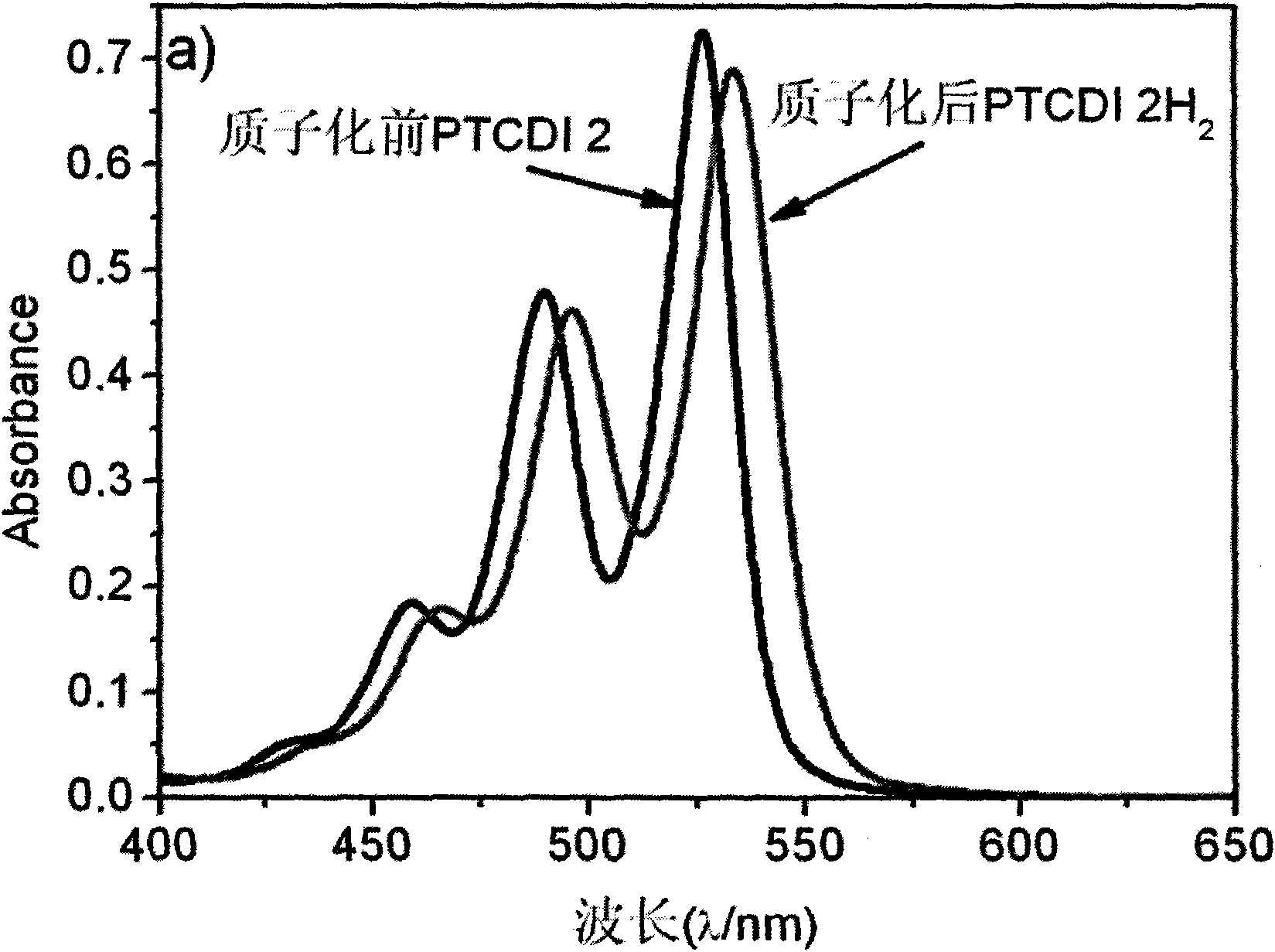
[0053] PTCDI 2 at a concentration of 1.0 x 10 -5 In the chloroform solution of M, trifluoroacetic acid is added to protonate the imine group, and the A of PTCDI 2 before and after protonation 0-0 / A 0-1 The ratio of PTCDI2 is basically unchanged, indicating that PTCDI2 exists in the form of monomer in chloroform solution before and after protonation, but the intensity of its fluorescence spectrum is enhanced by nearly 4 times, which is because the imino group on PTCDI2 is no longer an electron donor after protonation. body, intramolecular photoinduced electron transfer is blocked, so the fluorescence is enhanced ( image 3 ). The UV-Vis spectrum research under different pH values found that as the pH value decreased, the UV-Vis spectrum of PTCDI 2 changed significantly, and the peak shape narrowed, 0-0 peak (530nm) and 0-1 peak (496nm ) increased significantly, but the 0-0 peak increased much more than the 0...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com