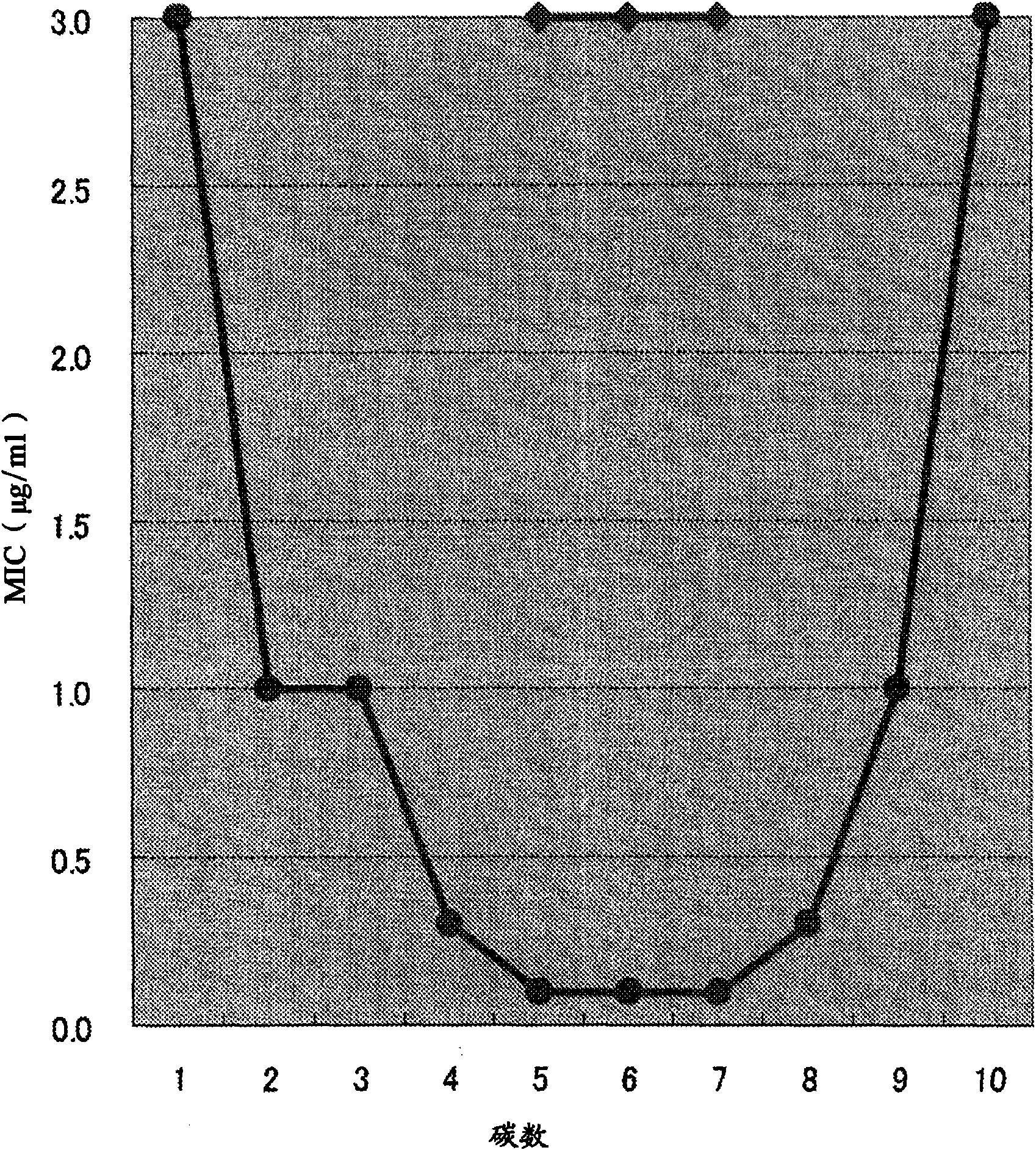
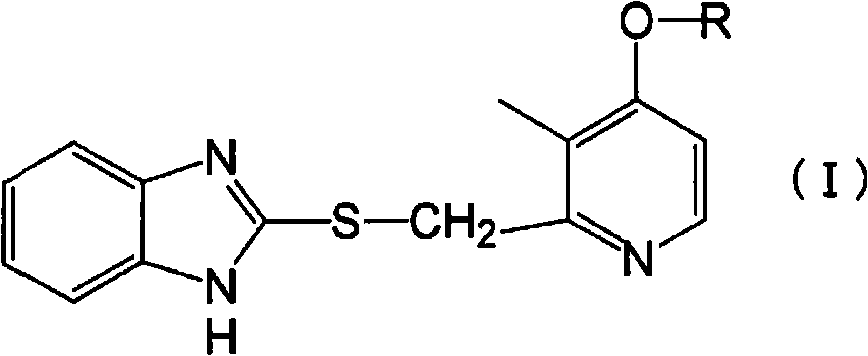
Helicobacter pylori eradicating agent having inhibitory activity on gastric acid secretion
A Helicobacter pylori, general formula technology, applied in the direction of anti-inflammatory agents, antibacterial drugs, organic active ingredients, etc., can solve the problems of unconfirmed effectiveness and difficulty in exerting antibacterial activity, and achieve excellent anti-Helicobacter pylori and gastric acid inhibition secretion effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0108] Synthesis Example 1: Preparation of 4-methoxy-2,3-lutidine-1-oxide
[0109] Dilute 41.6g (2.0 equivalents) of 28% sodium methoxide aqueous solution with 200ml of dimethyl sulfoxide, and add dropwise 17.0g of 4-chloro-2,3-lutidine-1-oxide dissolved in di solution after methylsulfoxide (70ml). After reacting at 40° C. to 50° C. for 3 hours, it was left to stand overnight at room temperature. 15ml of water was added to the reaction solution, and concentrated to obtain a black syrupy residue; then, after dissolving in 500ml of water, the aqueous solution was extracted three times with 670ml of chloroform. The extract was dried over anhydrous magnesium sulfate and then concentrated to dryness under reduced pressure to obtain 37.2 g of 4-methoxy-2,3-lutidine-1-oxide.
Synthetic example 2
[0110] Synthesis Example 2: Preparation of 2-acetoxymethyl-3-methyl-4-methoxy-pyridine
[0111] Add 55.0 g (5.0 equivalents) of acetic anhydride to 37.2 g of 4-methoxy-2,3-lutidine-1-oxide, and react at 90°C to 100°C for 3 hours. The concentrated residue obtained after distilling off acetic anhydride was purified with a silica gel column to obtain 15.8 g of oily 2-acetoxymethyl-3-methyl-4-methoxy-pyridine.
Synthetic example 3
[0112] Synthesis Example 3: Preparation of 2-hydroxymethyl-3-methyl-4-methoxy-pyridine
[0113] In 25% aqueous sodium hydroxide solution, 15.8 g of 2-acetoxymethyl-3-methyl-4-methoxy-pyridine was added dropwise, and after reacting at room temperature for 1 hour, it was diluted with toluene, and then the toluene phase was washed with water, After drying over anhydrous magnesium sulfate, it was concentrated to dryness to obtain 5.7 g of oily 2-hydroxymethyl-3-methyl-4-methoxy-pyridine.
PUM
| Property | Measurement | Unit |
|---|---|---|
| composition ratio | aaaaa | aaaaa |
| residual percentage | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com