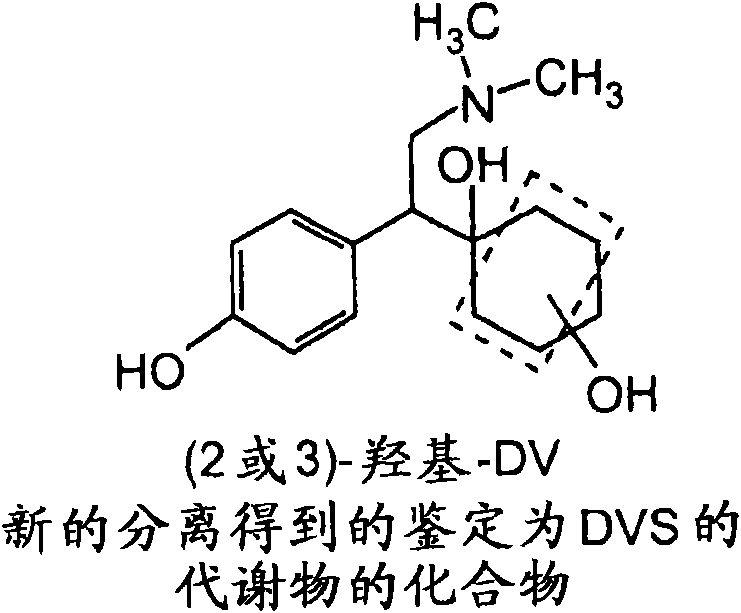
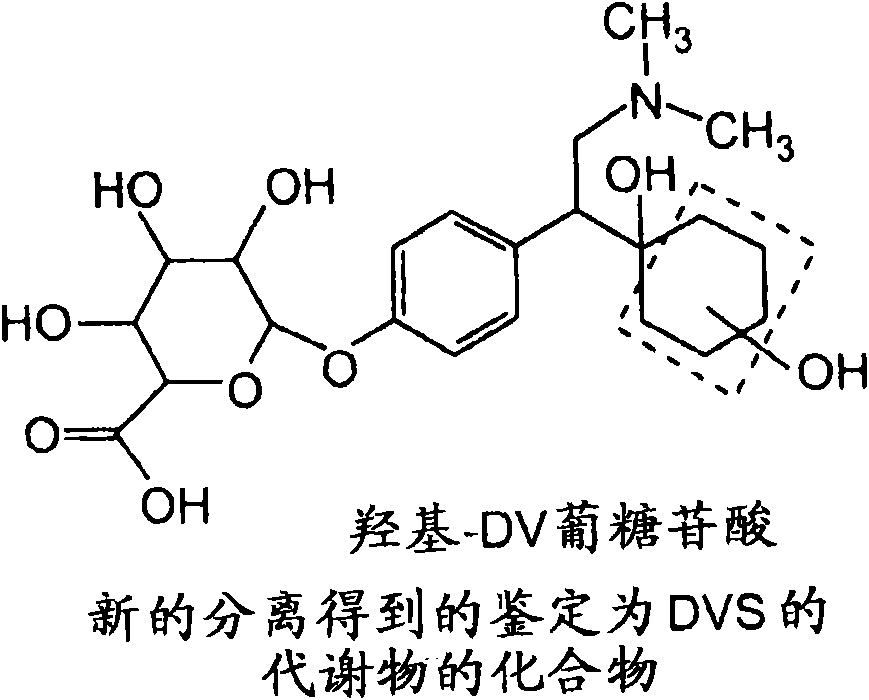
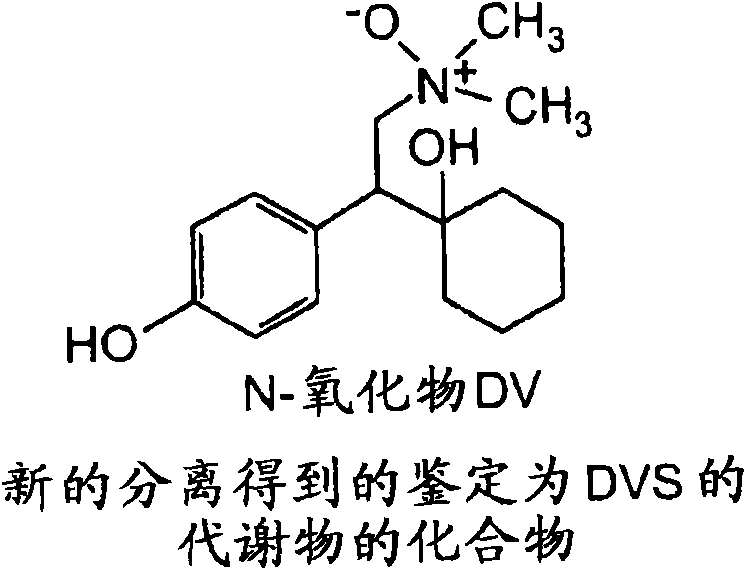
Isolated hydroxy and N-oxide metabolites and derivatives of O-desmethylvenlafaxine and methods of treatment
A technology for metabolites and derivatives, applied in the field of isolated O-desmethylvenlafaxine hydroxyl and N-oxide metabolites and derivatives and therapeutics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 5
[0061] C. Synthesis
[0062] 1. Synthesis of Free Base Compounds
[0063] The compounds of the present invention can be prepared by those skilled in the art using the methods described below, as well as synthetic methods known in the art of organic synthesis, or modifications of these methods. In general, see Comprehensive Organic Synthesis, "Selectivity, Strategy & Efficiency in Modern Organic Chemistry", edited by I. Fleming, Pergamon Press, New York (1991); Comprehensive Organic Chemistry, "The Synthesis and Reactions of Organic Compounds", edited by J.F. Stoddard, Pergamon Press, New York (1979). Suitable methods include, but are not limited to, those listed below.
[0064] figure 2 A method of synthesizing the 2-hydroxy DV compound of the present invention is provided. In the first step of this synthesis, 4-(demethylcarbamoylmethyl)phenol is protected with a benzyl group. The bromobenzyl protecting group is very suitable for use in the methods of synthesizing the com...
Embodiment 1
[0112] Example 1. In Sprague Dawley rats after oral administration alone [ 14 C] Metabolism of DVS
[0113] Following individual oral gavages to male and female rats as described below, in urine, feces, and plasma, [ 14 C] Six hydroxyl DV compounds and N-oxide DV compounds, among others, were detected in the metabolic profile of DVS.
[0114] Radioactively labeled [ 14 C] DVS (Lot #CFQ13003, [Cyclohexyl-1- 14 C] DVS) was provided by Amersham Biosciences (Buckinghamshire, UK). Unlabeled DVS (lot RB1636; free base 65.2%) was obtained from Wyeth Research, Rouses Point, NY. The average molecular weight of DVS is 381.5, and O-desmethylvenlafaxine accounts for 69.0% by weight. As determined by HPLC with radioactive detection, [ 14 C] The specific activity of DVS (crude drug) is 144 μCi / mg (209 μCi / mg for the free base), and the radiation purity of the free base is 99.3%.
[0115]Water used to prepare solutions for oral administration was obtained from EM Science (Gibbstown, N...
Embodiment 2
[0178] [ 14 Metabolism of C]O-desmethylvenlafaxine
[0179] Following oral gavage alone in male beagle dogs, [ 14 C] (2 or 3)-hydroxy DV compounds, hydroxy DV glucuronides, N-oxide DV compounds, among others, and benzyl hydroxy compounds were detected in the metabolic profile of DVS.
[0180] Materials and methods
[0181] Radiolabeled [ 14 C] DVS (Lot #CFQ13003, [Cyclohexyl-1- 14 C] DVS) was provided by Amersham Biosciences (Buckinghamshire, UK). Unlabeled DVS (lot RB1636; free base 65.2%) was from Wyeth Research, Rouses Point, NY. The average molecular weight of DVS is 381.5, of which the free base O-desmethylvenlafaxine accounts for 69.0% by weight. Measured by HPLC using radioactive detection method, [ 14 C] The specific activity of DVS (crude drug) is 144 μCi / mg (209 μCi / mg for the free base), and the radiation purity of the free base is 99.3%.
[0182] Water used to prepare solutions for oral administration was obtained from EM Science (Gibbstow, NJ). Methylcell...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com