Activity enhancer for detoxifying enzyme
A technology for detoxification enzymes and enhancers, which is used in organic active ingredients, animal feed, food preparation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0085] Preparation Example 1: Preparation of Agarobiose
[0086] Agar (AGAR NOBLE) was suspended in 0.1N HCl to a concentration of 10%, and then heated at 100° C. for 19 minutes. 10 ml of the above sample was loaded onto a water-equilibrated Toyopearl HW40C (manufactured by TOSOH Corporation) column (4.4 cm x 85 cm in volume). Separation was performed by gel filtration chromatography using water as mobile phase at a flow rate of 1.4 ml per minute. The eluted material was detected by differential refractometer and 7 ml fractions were collected.
[0087] Peaks appeared at elution times of 406 minutes, 435 minutes, 471 minutes and 524 minutes, respectively. Take the fractions corresponding to each peak, in silica gel 60 F 254 Plates (manufactured by Merck) were spotted, developed using 1-butanol:ethanol:water at a ratio of 5:5:1, and then analyzed using the orcin-sulfuric acid method. As a result, the peak eluted at 524 minutes was agarobiose. This fraction was lyophilized t...
preparation Embodiment 2
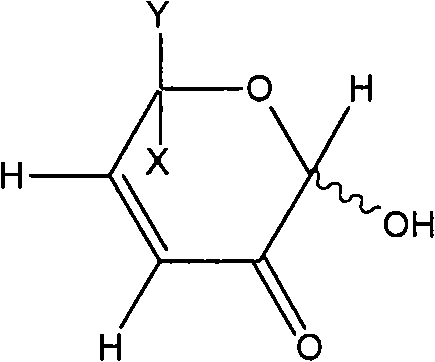
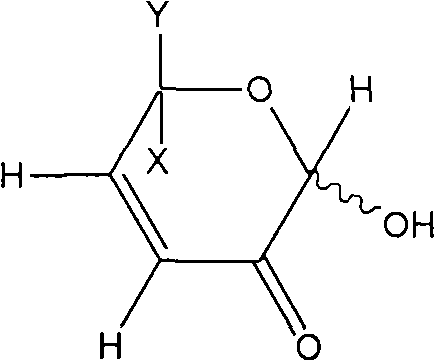
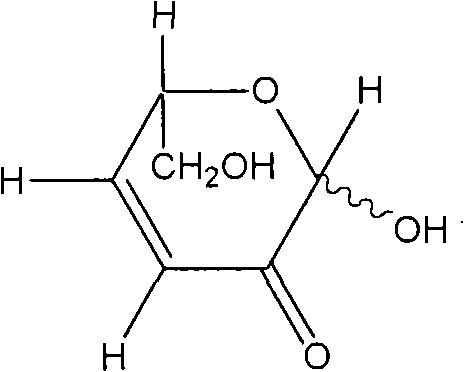
[0088] Preparation Example 2: Preparation of L-glycerol-1,5-epoxy-1αβ,6-dihydroxy-cis-hex-3-en-2-one (DGE)
[0089] A suspension of 2.5 g of commercially available agar (AGAR NOBLE) in 50 ml of 0.1N HCl was heated at 100° C. for 13 minutes to obtain a solution. The solution was cooled to room temperature, the pH was adjusted to 12 using NaOH, and then the solution was neutralized.
[0090] The neutralized product was purified using normal phase HPLC as described below. Each peak was collected, dried under reduced pressure, and redissolved in water. The cancer cell growth inhibitory activity of each fraction was measured by using HL-60 cells. Fractions with retention times of 4.05 and 4.16 were found to have cancer cell growth inhibitory activity.
[0091] Then, fractions with retention times 4.05 and 4.16 were collected in large quantities and subjected to structural analysis. This fraction was found to be L-glycerol-1,5-epoxy-1αβ,6-dihydroxy-cis-hex-3-en-2-one (DGE). Con...
Embodiment 1
[0099] Example 1: Evaluation of the effect of enhancing glutathione S-transferase (GST) activity (1)
[0100] Hepa1c1c7 cells (ATCC CRL-2026) were suspended in Dulbecco's modified Eagle's culture containing 10% fetal bovine serum (manufactured by MP Biomedicals) and 1% penicillin-streptomycin (manufactured by NacalaiTesque Co., Ltd.) base (Dulbecco modified Eagle medium) (manufactured by Sigma), the cell concentration is 4 × 10 5 cells / ml. Into each well of a 96-well microtiter plate, 0.2 ml of cell suspension was added, and cultured overnight at 37° C. in the presence of 5% carbon dioxide gas. Then, replace the medium in each well with Dulbecco's modified Eagle's medium. To each well, 0.4 µl of an aqueous solution of the test substance was added, followed by incubation for 24 hours. The agarobiose obtained in Preparation Example 1 and the commercially available agarooligosaccharide (commercial name: Agaoligo, manufactured by Takara Bio Co., Ltd., both containing 20 to 25% ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com