Prodrug based on gemcitabine structure as well as synthesizing method and application thereof
A technology of gemcitabine and synthesis method, applied in the field of nucleoside drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
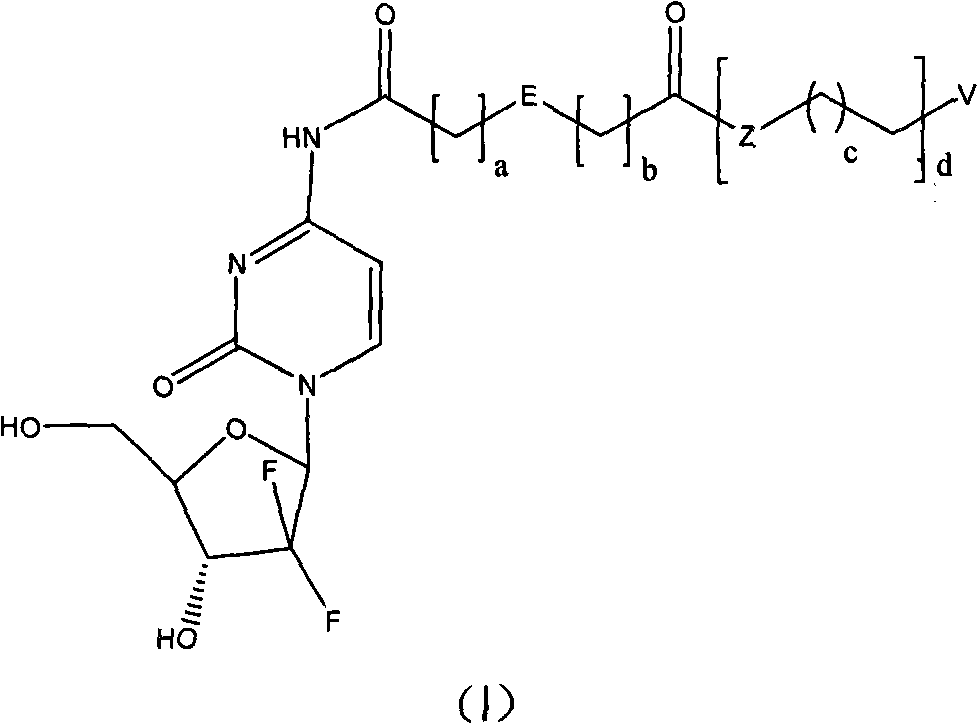
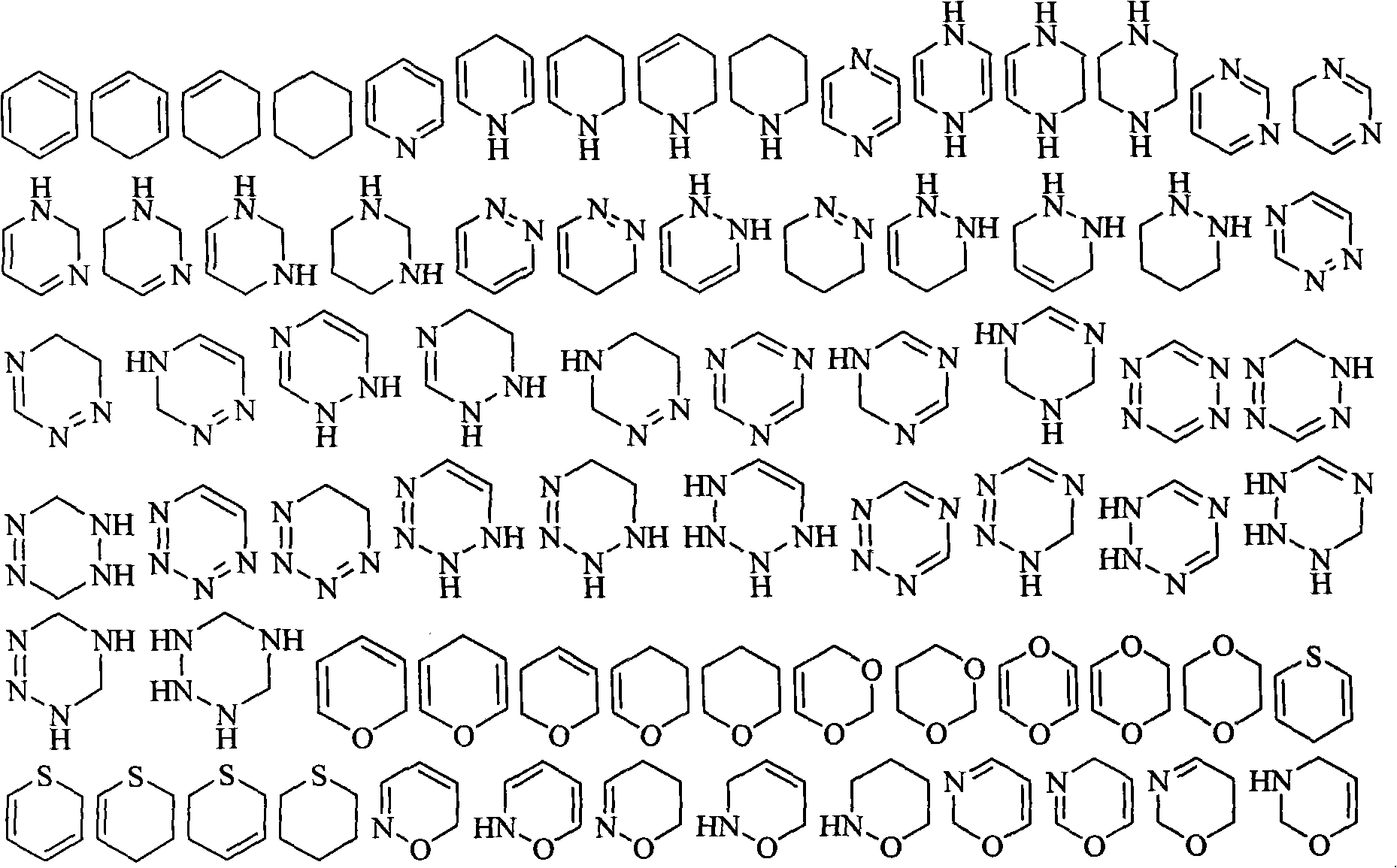
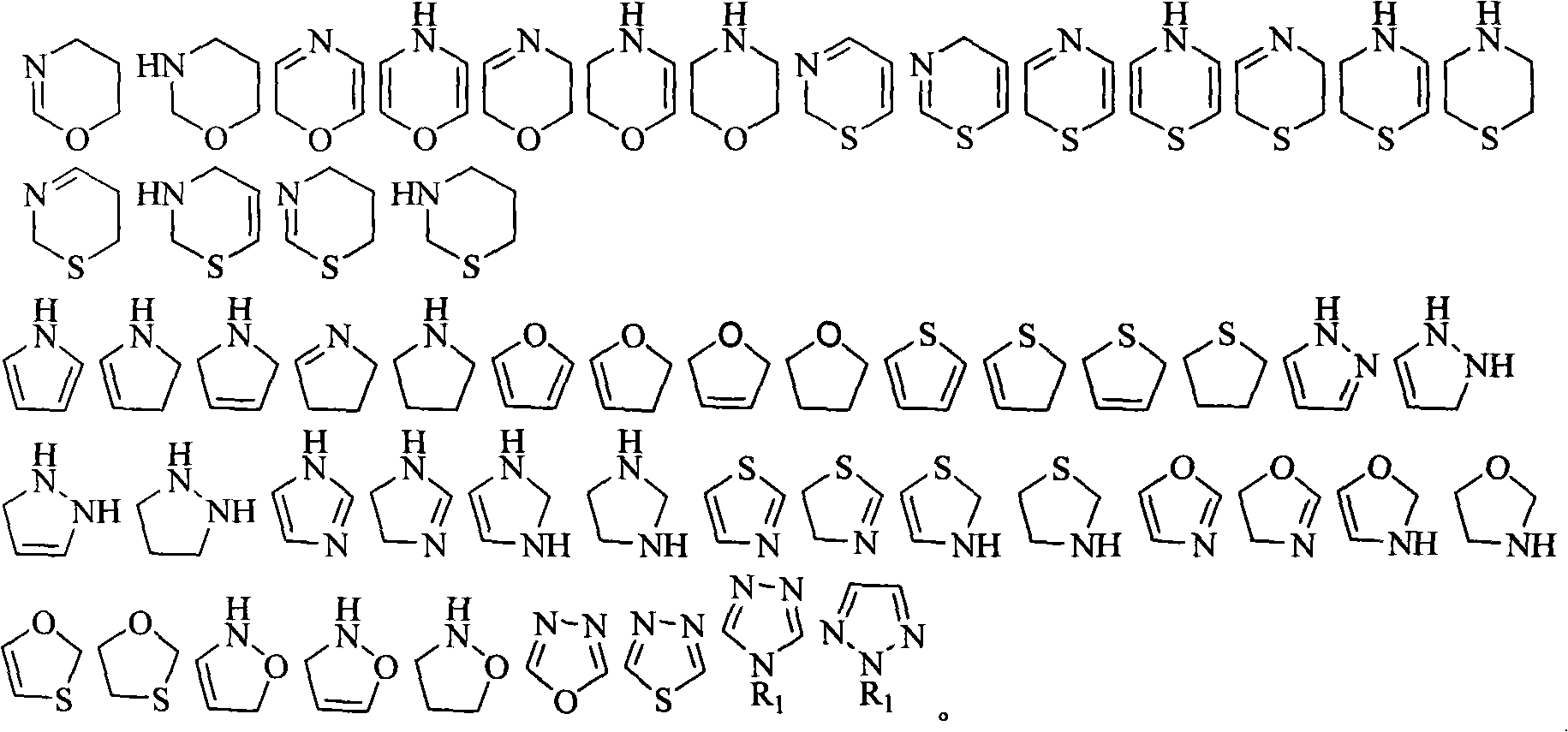
[0055] The present invention also provides the synthetic method of the prodrug based on the gemcitabine structure, comprising the following steps:
[0056] 1) The acid anhydride or acid chloride and alcohol or amine are directly mixed or dissolved in an organic solvent according to the ratio of acid anhydride or acid chloride: alcohol or amine = 1: 1 ~ 1: 1.5 molar ratio, and react at room temperature to the melting temperature of the reactant for 2 ~ 8 hours, the corresponding substituted acid is obtained;
[0057] 2) Gemcitabine hydrochloride, the substituted acid obtained in step 1), benzotriazole-1-oxyl tripyrrolidinylphosphonium hexafluorophosphate and 4-dimethylaminopyridine according to 1: (1~2):( 0.9~1.5): (1~3) molar ratio is dissolved in the organic solvent, stirred at room temperature for 12~24 hours;
[0058] 3) In step 2), the reaction solution is poured into water, extracted, and the separated organic phase is dried and purified to obtain the target product.
...
specific Embodiment approach
[0088] Hereinafter, the present invention will be described more specifically using examples, but the present invention is not limited to the following examples.
[0089] Gemcitabine hydrochloride was purchased from Ningbo Tianheng Pharmaceutical Co., Ltd.;
[0090] Benzotriazole-1-oxytripyrrolidinylphosphine hexafluorophosphate was purchased from Jill Biochemical (Shanghai) Co., Ltd.;
[0091] 4-Dimethylaminopyridine was purchased from Jill Biochemical (Shanghai) Co., Ltd.
Embodiment 1
[0093] N 4 -(2-Dodecyloxyformylbenzoyl)-2'-deoxy-2',2'-difluorocytidine (Compound NO.1)
[0094] Mix 5.0g phthalic anhydride (34mmol), 7.5g lauryl alcohol (40mmol) and 240mg 4-dimethylaminopyridine (2mmol), heat and melt, react for 4 hours, cool to room temperature, and quantitatively obtain 2-n- Lauryloxyformylbenzoic acid.
[0095] With 700mg gemcitabine hydrochloride (2.34mmol), 1.02g 2-n-dodecyloxyformylbenzoic acid (3.04mmol), 1.34g benzotriazole-1-oxyl tripyrrolidinylphosphonium hexafluorophosphate ( 2.57mmol) and 428mg of 4-dimethylaminopyridine (3.51mmol) were dissolved in N,N-dimethylformamide (10mL), and stirred overnight at room temperature. The reaction solution was poured into water, extracted three times with ethyl acetate, and the separated organic phase was dried over anhydrous sodium sulfate. After removing the solvent by evaporation under reduced pressure, the residue was purified on a silica gel column (developing solvent: dichloromethane / methanol=30 / 1) t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com