Method for synthesizing D-mannoheptulose
A technology of mannoheptulose and mannoheptulose, which is applied in the field of synthesis of D-mannoheptulose, can solve the problems of cumbersome transformation methods and achieve the effects of low production cost, mild conditions and short process route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
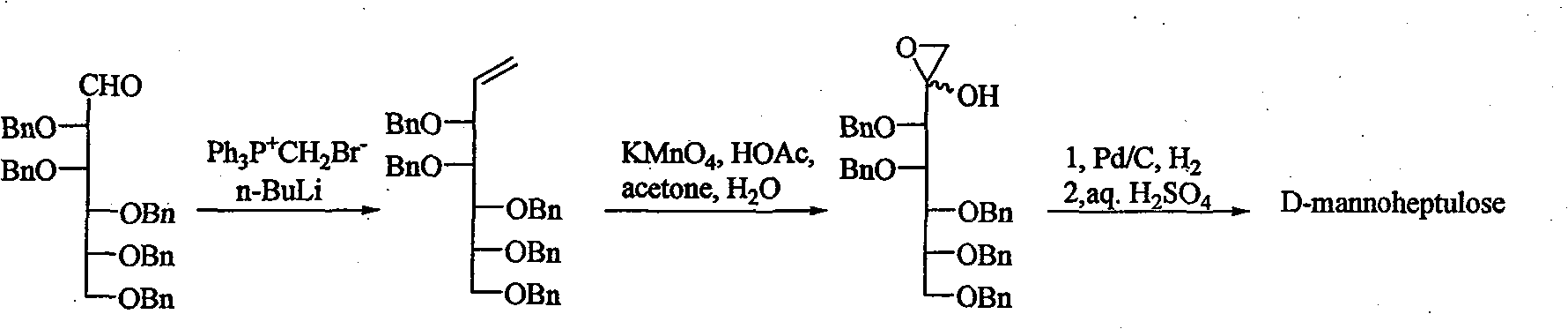
[0017] The invention discloses a method for synthesizing D-mannoheptulose, comprising the following steps:
[0018] Step 1: Preparation of 3,4,5,6,7-penta-O-benzylmannohepsene,
[0019] in N 2 Under the protection, anhydrous toluene was added to triphenylmethyl phosphine bromide, under ice bath, the n-hexane solution of n-butyllithium was added dropwise to the system, the temperature was increased, and 2,3 dissolved in anhydrous toluene was added. , 4,5,6-penta-O-benzylmannose, add acetone and water to terminate the reaction after the reaction, extract, wash, dry, concentrate, silica gel column chromatography to obtain 3,4,5,6,7-penta -O-benzylmannohepsene;
[0020] In the above-mentioned process, the Wittig reagent made by triphenylmethyl phosphine bromide and n-butyllithium needs to be excessively higher than 2,3,4,5,6-penta-O-benzyl mannose, and the two dosages are relative to The dosage of 2,3,4,5,6-penta-O-benzylmannose is not less than 2 times the molar equivalent; wh...
Embodiment 1
[0029] In a 100mL there-necked flask, add 12.8g (35.7mmol) triphenylmethyl phosphine bromide, N 2 Add 35 mL of anhydrous toluene under protection and stir well. Under the ice bath, 23mL of 1.6mol / L BuLi n-hexane solution was added dropwise, and the temperature was increased to 35°C after 40min. At this time, the reaction system was dark yellow. 4,5,6-Penta-O-benzylmannose, reacted at 28°C for 2d. Add 10 mL of acetone and 10 mL of water to stop the reaction. Concentrated, 80mL CH 2 Cl 2 Dissolved, washed with 3×200mL water in turn, Na 2 SO 4 Drying, filtration, concentration, silica gel column chromatography (V (ethyl acetate): V (petroleum ether) = 1:8 elution) to obtain 5.8 g of pale yellow syrupy 3,4,5,6,7-penta- O-benzylmannoheptenene (88% yield).
[0030] Add 4.54g (7.2mmol) 3,4,5,6,7-penta-O-benzylmannoheptonene to a 250mL round-bottomed flask, add 70.3mL acetone, 13.9mL water, 3.2mL acetic acid to dissolve, ice bath Mix well. 23.0mL of acetone and 1.80g (11.4mmo...
Embodiment 2
[0033] Add 12.6g (35.3mmol) triphenylmethylphosphine bromide in 100mL there-necked flask, N 2 Add 40mL of anhydrous toluene under protection and stir well. Under an ice bath, add 33 mL of 1.2 mol / L BuLi n-hexane solution dropwise, and heat up to 25°C after 1 hour. At this time, the reaction system appears dark yellow. Add 8.0 g (12.7 mmol) of 2,3 dissolved in 10 mL of anhydrous toluene dropwise, 4,5,6-penta-O-benzylmannose, react at room temperature for 36h. 10 mL of acetone and 10 mL of water were added to stop the reaction. Concentrate, 80mLCH 2 Cl 2 Dissolved, washed with 3 × 200mL water successively, Na 2 SO 4 Drying, filtration, concentration, and silica gel column chromatography (V (ethyl acetate): V (petroleum ether) = 1: 8 elution) gave 6.7 g of light yellow syrupy 3,4,5,6,7-penta- O-Benzylmannoheptosene (82% yield).
[0034] Add 5.60g (8.9mmol) 3,4,5,6,7-penta-O-benzylmannoheptosene to a 250mL round bottom flask, add 86.3mL acetone, 13.9mL water, 4.2mL acetic a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com