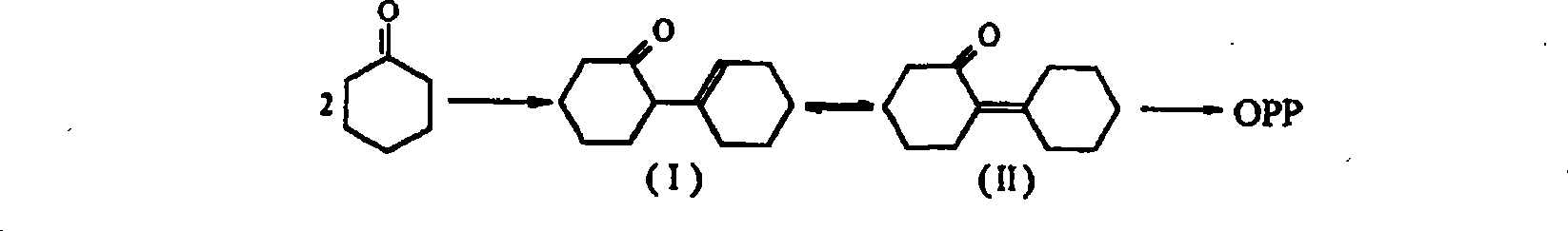
O-phenyl phenol preparation catalyst by cyclohexanone dimer dehydrogenation and preparation method thereof
A technology of cyclohexanone dimer and o-phenylphenol is applied in the field of catalyst and preparation field for preparing o-phenylphenol by dehydrogenation of cyclohexanone dimer, and can solve the problem of low conversion rate and selectivity, low catalyst The problems of poor stability and high catalyst cost can achieve the effect of simple activation and regeneration procedure, improved structural stability, high yield and selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Take by weighing 1300 grams of pseudo-boehmite and 1000 grams of water and mix and stir to make pseudo-boehmite slurry, after stirring, add 82 grams of lanthanum nitrate hexahydrate (La(NO) dissolved in 600 milliliters of deionized water while stirring. 3 ) 3 ·6H 2 O) Aqueous solution, stirred to fully dissolve, after uniform dispersion, nitric acid solution was added dropwise, degummed until the pseudo-boehmite slurry became a sol state, the sol was dried at 100-120°C, and then dried at 500°C Calcined for 6 hours, and after cooling down, it was developed into 20-40 mesh particles to obtain lanthanum oxide-modified alumina (La 2 o 3 / γ-Al 2 o 3 ).
Embodiment 2
[0038] Weigh 0.148 grams of potassium sulfate and dissolve it in 6 ml of deionized water, get 8 grams of catalyst carrier in Example 1 and soak for 4 hours, then dry at a constant temperature at 100 to 120° C. for 10 hours, and roast at 500° C. for 6 hours; measure 0.02 grams / ml of chloroplatinic acid (H 2 PtCl 6 ·6H 2 O) 3.19ml of solution was added with deionized water to make 6ml of solution, and the previously calcined catalyst was impregnated therein for 4 hours, then dried at a constant temperature at 100-120°C for 10 hours, and calcined at 500°C for 6 hours. Finally, the catalyst was activated with hydrogen diluted with nitrogen, the flow rate of nitrogen gas was 60 ml / min, and the flow rate of hydrogen gas was 6 ml / min. Under the pressure of 0.1MPa, the reduction activation was carried out in the fixed bed reactor at 380°C for 6 hours until there was no water vapor at the outlet of the reactor, and it was cooled for later use.
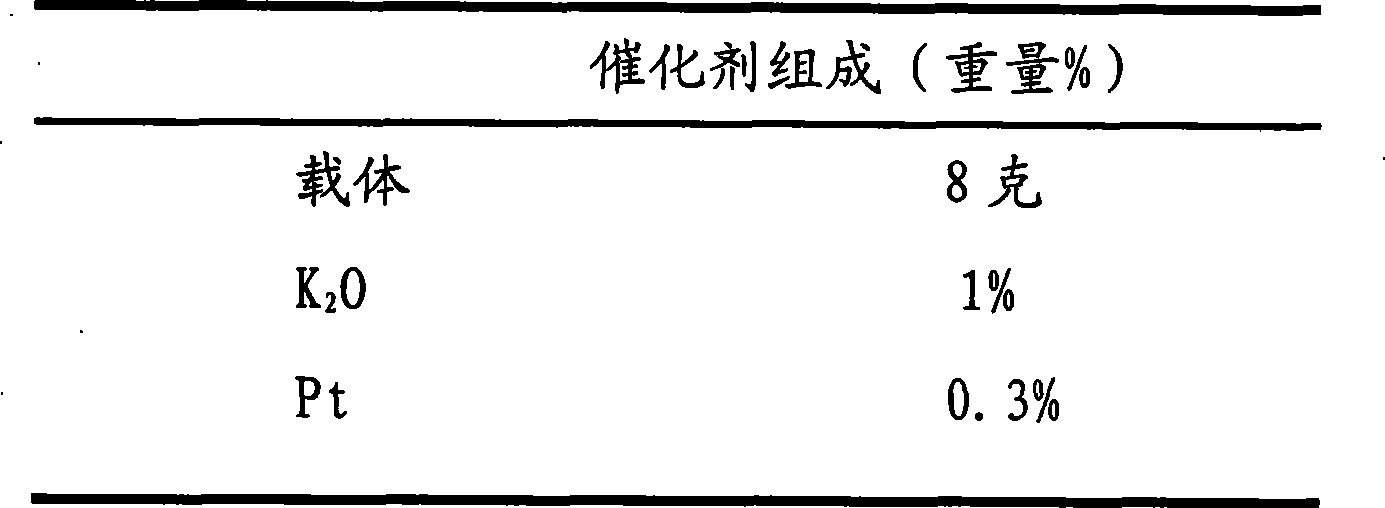
[0039] The composition of catalyzer ...
Embodiment 3
[0043] Except that the amount of potassium sulfate is changed to 0.296 grams, and the chloroplatinic acid solution is changed to 4.26 ml. Catalyst preparation conditions are the same as in Example 2.
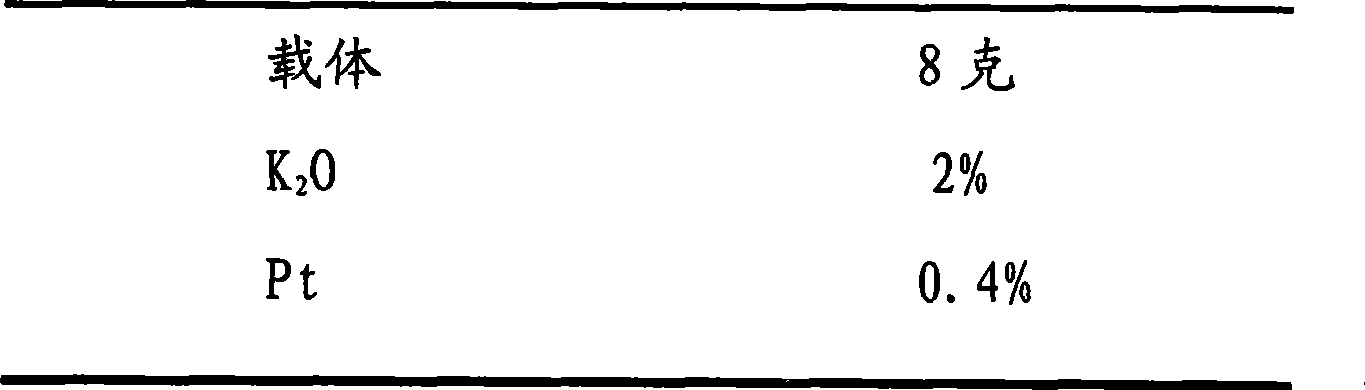
[0044] The composition of catalyzer is as follows: (accounting for carrier percent weight by active component)
[0045] Catalyst composition (weight%)
[0046]
[0047] The catalyst performance evaluation results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com