Nematophagous fungi infectious extracellular serine proteinase crystal morphology
A technology of serine protease and nematophagous fungi, which is applied in the fields of molecular biology and applied microbiology, and can solve the problems of undiscovered public reports and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1: Structural analysis of the extracellular serine protease from Verticillium lanceolata Lecanicillium psalliotae
[0036] Inoculate the strain of Verticillium psalliotae (Lecanicillium psalliotae, YMF1.00112) with the preservation number CGMCC No. 1312 cultured on the PDA plate in the enzyme production medium, and culture it on a shaker at 28° C. at 150 rpm for 6 days. The fermentation broth was filtered and the supernatant was precipitated with ammonium sulfate. Fractions having protease activity were dissolved in 10 mM PBS (pH 6.0) buffer and purified by cation exchange chromatography. The enzymatically active eluate was further purified by molecular sieves. The electrophoretic pure protease Ver112 was obtained, concentrated and then crystallized to obtain protease crystals. The three-dimensional structure of the protease was obtained by X-ray diffraction of the crystal and processed with software such as HKL2000 and ccp4.
Embodiment 2
[0037] Example 2: Structural Analysis of Paecilomyces lilacinus Extracellular Serine Protease
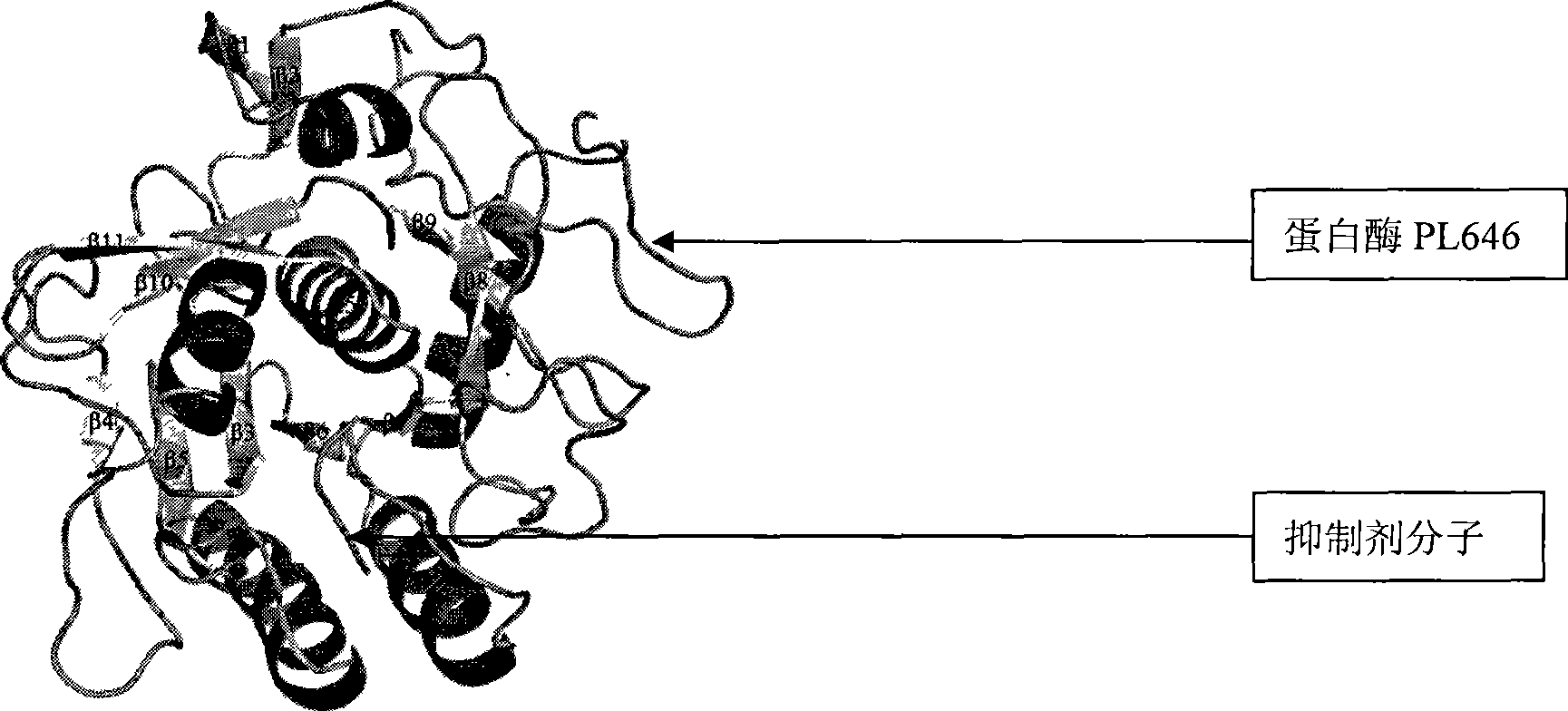
[0038] Paecilomyces lilacinus (M-14) cultured on the PDA plate, the preserved strain of CGMCC No0241. was inoculated in the enzyme production medium, and cultured on a shaker at 28° C. at 150 rpm for 6 days. The fermentation broth was filtered and the supernatant was precipitated with ammonium sulfate. The protease active fraction was dissolved in 10 mMPBS (pH 6.0) buffer and purified by cation exchange chromatography. The enzymatically active eluate was further purified by molecular sieves. The electrophoretic pure protease PL646 was obtained, concentrated and then crystallized to obtain protease crystals. The three-dimensional structure of the protease was obtained by X-ray diffraction of the crystal and processed with software such as HKL2000 and ccp4.
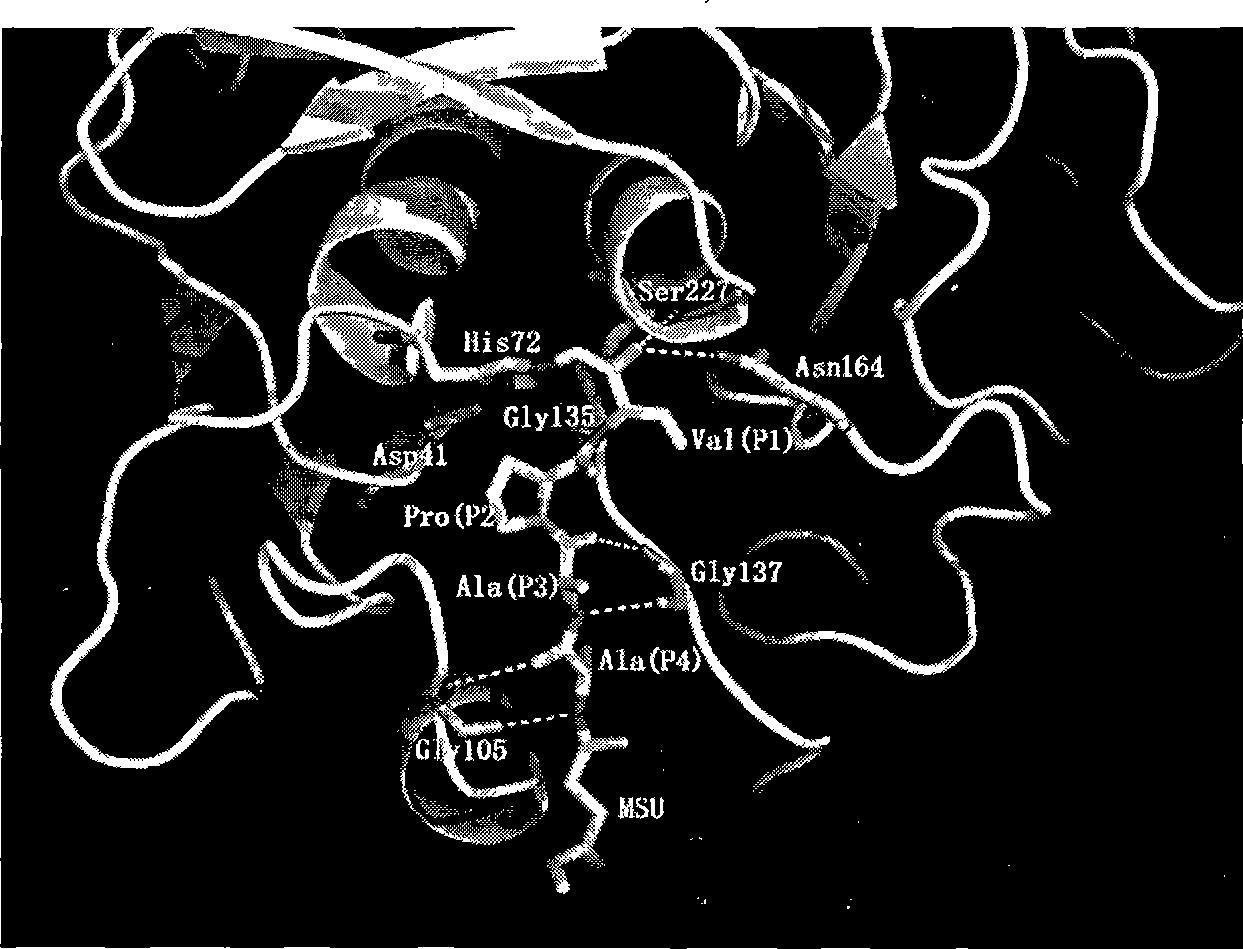
[0039] The crystal structure of the extracellular alkaline serine protease of the nematode fungus obtained by the above-ment...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com