Infectivity-enhanced conditionally-replicative adenovirus and uses thereof
a technology of adenovirus and replicative vector, which is applied in the field of adenovirus vectors, can solve the problems of poor infectability of primary tumors, affecting the efficiency of replicative vectors, and clinical trials that are less than optimal, so as to increase the infectivity of adenoviruses, improve the efficiency of replicative vectors, and improve the infectivity of primary tumors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Enhanced Tumor Transduction with Adenoviral Vectors Modified with an Antibody Conjugate
[0078]As a first approach towards enhancing the infectivity of adenoviral vectors and to demonstrate the tumor transduction advantage of vectors with altered tropism over unmodified vectors, an anti-fiber antibody conjugated to fibroblast growth factor (FGF2) was used. The Fab portion of the anti-knob antibody, 1D6.14, which is capable of blocking the interaction of the fiber with its cognate cellular receptor, was chemically conjugated to FGF2. The resulting Fab-FGF2 conjugate was complexed with adenoviral vectors expressing luciferase or β-galactosidase reporter genes to compare the transduction efficiency of the modified and unmodified vectors. Vector modification increased the level of gene expression more than 9-fold, as measured by luciferase activity (FIG. 1A), largely due to transduction of a greater percentage of target cells as seen by β-galactosidase staining (FIG. 1B). This experiment ...
example 2
Genetic Modification of the Hi Loop of the Fiber Provides Enhanced Infectivity to Adenoviral Vectors
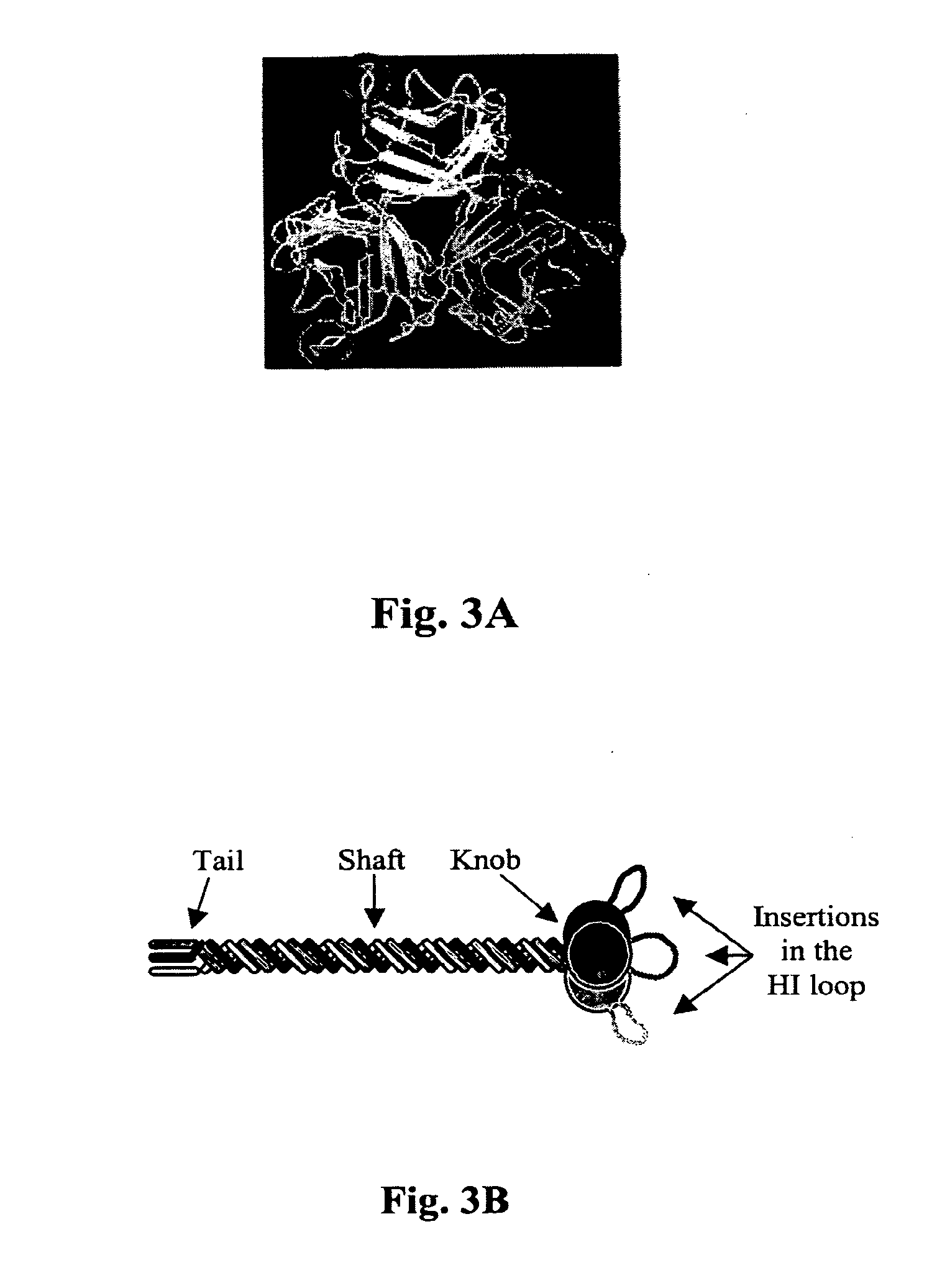
[0080]The Fab-ligand conjugation method described in Example 1 only modifies the tropism of the vector prepared for inoculation. In the context of a replicative vector, it is advantageous to modify the tropism of the vector that replicates in the tumor as well. With this rationale, a genetic modification of the fiber is necessary for replicative vectors because it is carried over to the progeny. As a simple and potent strategy for retargeting, the sequence of the fiber was genetically modified. Based on the three-dimensional model of the fiber knob, targeting ligands were inserted into the HI loop of the fiber (FIG. 3). This loop is flexible, exposed on the outside of the knob, is not involved in fiber trimerization and its variable length in different Ad serotypes suggests that insertions or substitutions would not affect the fiber stability.
[0081]As a ligand to introduce into the HI...
example 3
Enhanced Tumor Transduction Via RDG-Fiber Modification
[0085]To determine if the RGD sequence incorporated into the HI loop of the fiber could increase the infectivity of tumors, the ability of the modified vector to deliver genes to cultured human ovarian cancer cells was examined. Characterization of two cell lines, SKOV3.ipl and OV-4, by flow cytometry showed that they both express moderate-to-high levels of ανβ3 and ανβ35 integrins. SKOV3.ipl also expresses a high level of CAR, whereas OV-4 only modestly expresses CAR.
[0086]The incorporation of recombinant RGD-containing fiber protein in the Ad5lucRGD vector dramatically improved the ability of the virus to efficiently transduce these cells (FIG. 4A). At different MOIs tested, Ad5lucRGD-transduced cultures of SKOV3.ipl cells showed 30-fold to 60-fold increase in luciferase activity compared to cells transduced with control virus. Interestingly, while the purified fiber knob blocked over 90% of AdCMVLuc-mediated gene transfer, it ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com