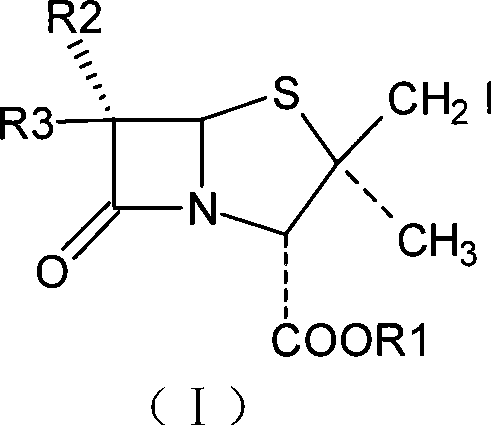
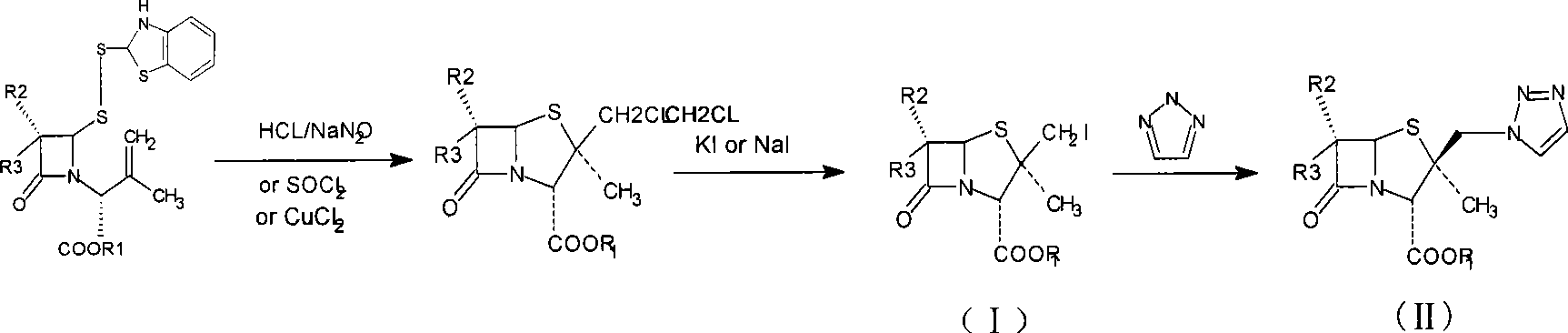
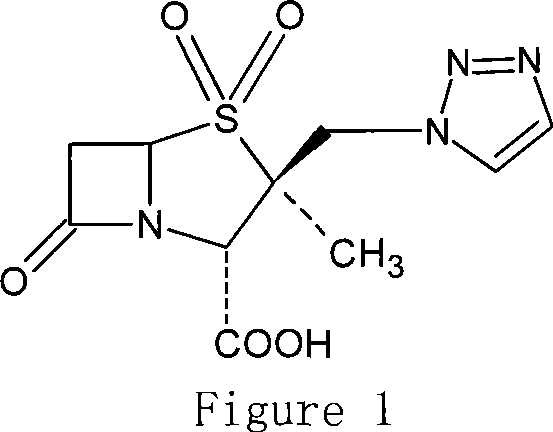
Penam iodide, preparation and use thereof
A technology of iodide and penicillanoate, applied in the field of chemistry, can solve the problems of tautomerism, limited industrial scale, complicated preparation and the like, and achieves the effects of convenient operation, control of production cost and high synthesis yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 13
[0039] Embodiment 1.3-Methyl-{2-oxo-4-(2-benzothiazole dimercapto)-1-azetidinyl}-butenoic acid diphenylmethyl ester
[0040] Add 500ml of toluene to a clean and dry reaction bottle equipped with a water separation device, heat the oil bath to reflux for 1 hour to remove water, and discard 50ml of toluene; when it is cooled to about 100°C, add 6,6-dihydropenicillane Acid diphenylmethyl ester-1-oxide 50g (0.13mol) and 2-mercaptobenzothiazole 21g (0.128mol), heating temperature to 130-140°C, reflux to separate water, control the reflux speed in the middle process, stir the reaction 2 ~3 hours later, the reaction was completed after sampling was monitored by HPLC. Reflux to drop about 400ml of toluene or water mixture, cool to 40°C, concentrate under reduced pressure, add 800ml of isopropyl ether to dissolve, stand in an ice bath for 20 hours to crystallize, precipitate a white solid, filter, and recover the mother liquor 65g of the product was obtained, the melting point was 78-...
Embodiment 22
[0041] The preparation of embodiment 2.2β-iodomethylpenicillanic acid diphenylmethyl ester
[0042]Add 36g (0.068mol) of the disulfide ring-opened product of Example 1 and 500ml of dichloromethane into a clean flask, stir until the solid is completely dissolved, cool to -5-0°C, add 10g of sodium nitrite (0.14mol) and 150ml of Sodium nitrite solution prepared with water was slowly added dropwise into 180 ml of 6N hydrochloric acid solution under vigorous stirring, and the reaction was monitored by HPLC. Control the reaction temperature at 0-5°C, react for 2 hours, let stand, and separate the organic phase; extract the water phase with 100ml of dichloromethane; combine the organic phases, wash with water, 10% sodium bicarbonate solution, and saturated sodium chloride Solution wash. Cool down to -5~0°C.
[0043] Dissolve 16.6g (0.10mol) of sodium iodide in 300ml of acetone, cool down to 5°C and add to the above dichloromethane feed solution. After stirring well, add 6g (0.10mol...
Embodiment 32
[0044] Example 3.2 Preparation of α-methyl-2β-(1,2,3-triazol-1-yl)methylpenicillane-3α-diphenylmethyl carboxylate-1,1-dioxide
[0045] The feed liquid of 2β-iodomethylpenicillanic acid diphenylmethyl ester obtained in Example 2 was cooled to 0° C., 85 ml of glacial acetic acid was added, and after stirring evenly, 20 g of potassium permanganate ((0.127 mol) was added in batches several times. , then reacted at room temperature for 3 to 4 hours.In the reaction solution, 30% hydrogen peroxide was added dropwise until the reaction solution became colorless, filtered, washed with a small amount of dichloromethane, and the organic phase was separated, and the aqueous phase was extracted with 100ml of dichloromethane; The dichloromethane phase is washed with water and saturated sodium chloride solution successively. The dichloromethane feed liquid containing product is obtained, and the dichloromethane is distilled out under reduced pressure, and filtered to obtain the crude product,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com