Fluorescent chemical sensor for furadantin detection
A chemical sensor, nitrofurantoin technology, applied in the field of fluorescent sensors, can solve the problems of loss of fluorescent carrier, failure to provide sensor analysis characteristics, etc., and achieve the effect of short fluorescent response time, simple structure and large amplitude
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0014] (1) Sample preparation
[0015] 1.0 g of the compound aminopyrene was dissolved in 15 ml of anhydrous DMF (dimethylformamide), and 0.60 ml of triethylamine was added. 0.40 ml of methacryloyl chloride was added dropwise under vigorous stirring, and after the drop was completed, the stirring reaction was continued at room temperature for 4 hours. After the reaction was finished, the precipitate generated was removed by filtration, the filtrate was collected, and the solvent was removed under reduced pressure to obtain 1.10 g of taupe solid methacrylamidopyrene, with a yield of 72.6%, M + =285.
[0016]
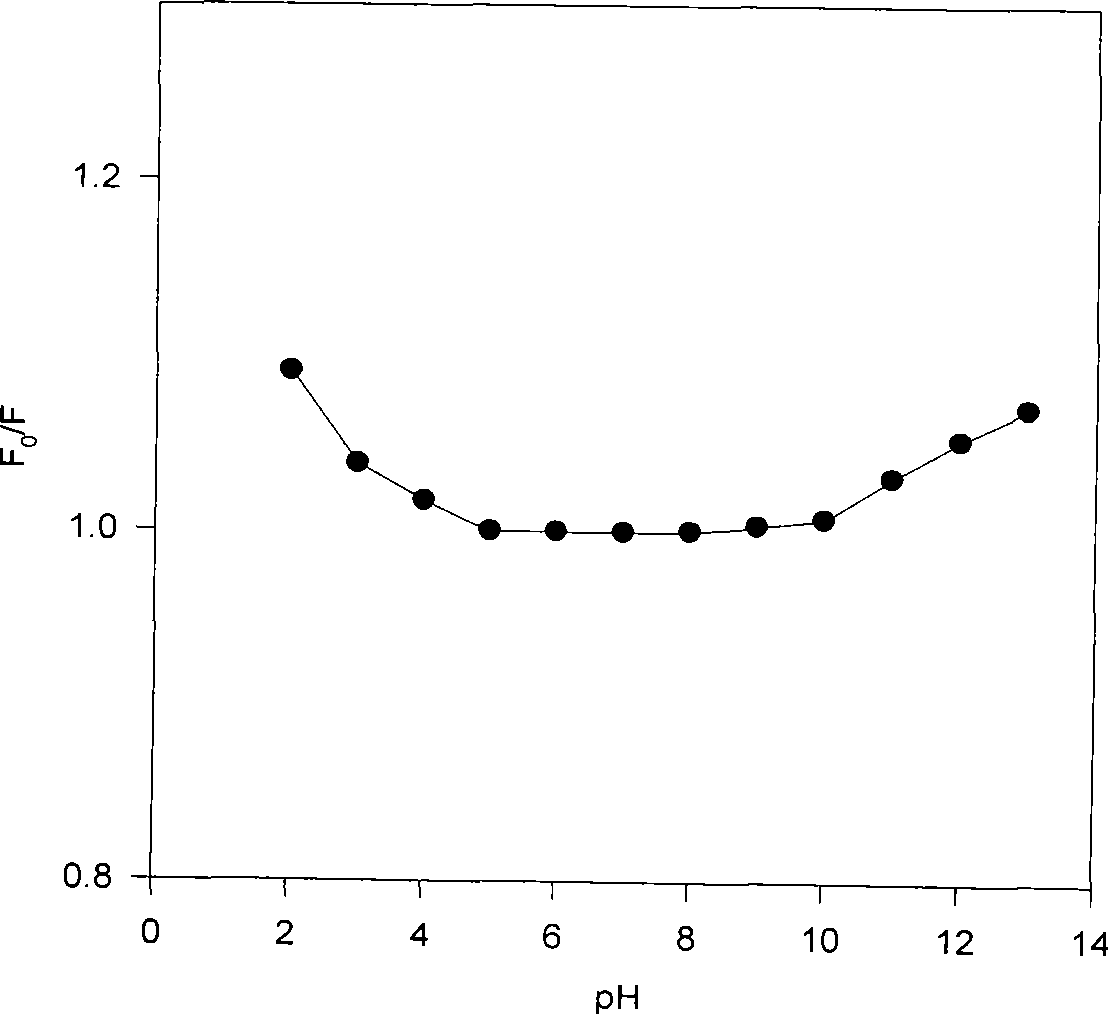
[0017] The fluorescent molecules of the methacrylamide-based pyrene photopolar film contain carbonyl groups and imino groups, which are easy to polarize and are sensitive to acidic solutions. Such as figure 2 As shown, the fluorescence intensity of the methacrylamido-pyrene optode film is greatly affected by pH when the pH is higher than 5.0 or lower than the pH is...
experiment example 1
[0023] Experimental example 1: Place the prepared sensing film in the detection cell, inject the sample solution into the detection cell with a syringe, measure the fluorescence intensity at the emission wavelength of 424 nm when the maximum excitation wavelength is fixed at 366 nm, and the recording film and the sample solution reach equilibrium fluorescence intensity at time. After each measurement, use a syringe to inject a blank solution to clean the methacrylamido-pyrene photoelectrode film, so that its fluorescence intensity returns to the initial value for the next measurement. Plotting the fluorescence response of different concentrations of nitrofurantoin solutions for the fluorescence intensity of the optode membrane Figure 4 , when the concentration of nitrofurantoin is 1.0×10 -6 -1.0×10 -4 mol l -1 When between, the calibration curve is shown in Figure 4 , the correction equation is F 0 / F=1.007+8142.200[A](r=0.9969), which can be used as a sensor to determi...
experiment example 2
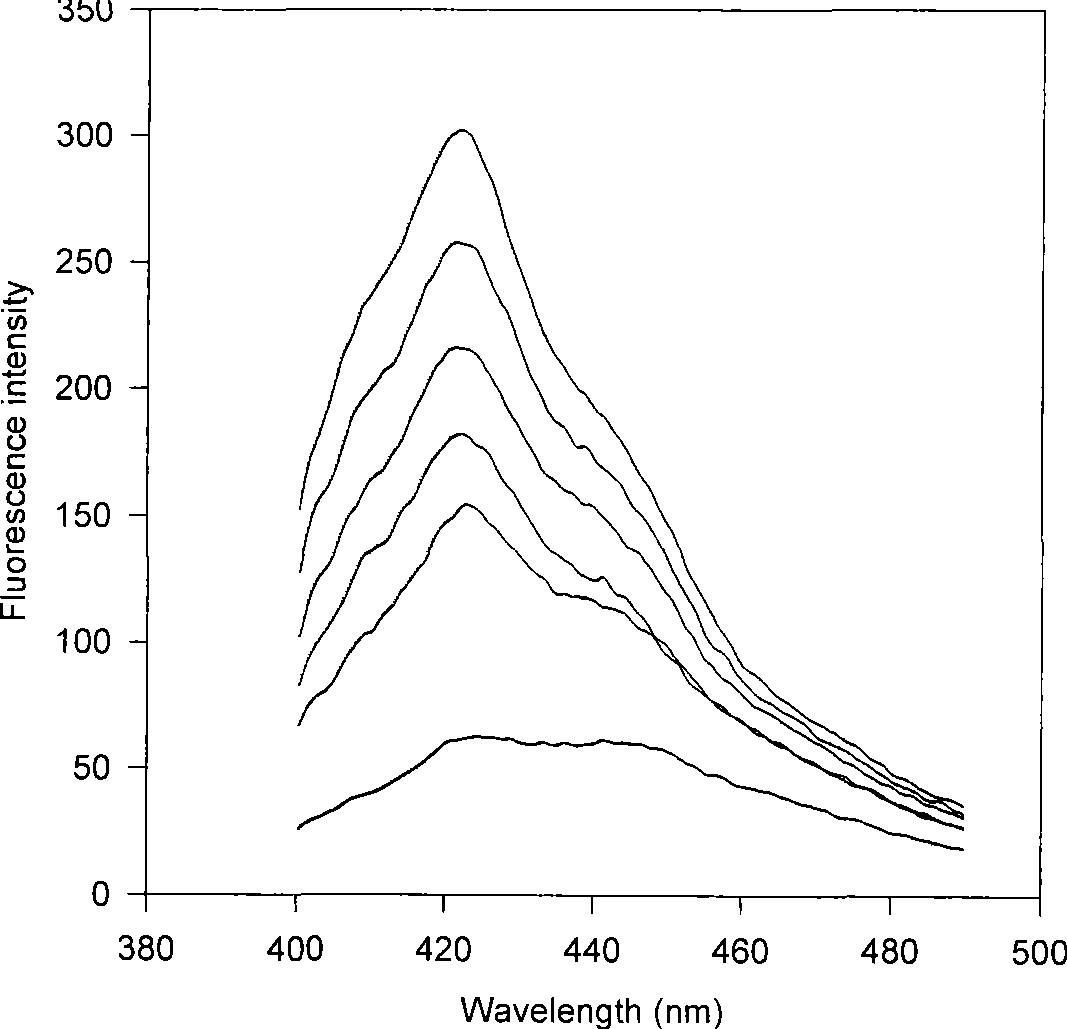
[0024] Experimental Example 2: Place the quartz glass slide attached with the optode film in the detection cell, and perform fluorescence measurement on a PerkingElmer LS55 fluorescence instrument with a computer data processing system. The light source is a 150W xenon lamp, and the detector is an R928F infrared-sensitive photomultiplier tube. The sample solution is injected into the detection cell with a syringe, and a stable fluorescence intensity value can be obtained after the optode membrane and the sample solution reach equilibrium. Fluorescence chemical sensor of the present invention can be applied to the mensuration of nitrofurantoin, such as figure 1 As shown, its emission spectrum is obtained by fixing the excitation wavelength at 366nm. The concentration of nitrofurantoin from top to bottom is (1) 0, (2) 4.0×10 -5 moll -1 , (3)6.0×10 -5 mol l -1 , (4)8.0×10 -5 mol l -1 , (5)1.0×10 -4 mol l -1 , (6)2.0×10 -4 mol l -1 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com