Method for preparing high-purity hydrochloride naphthodiamide
A naphthalene ethylenediamine hydrochloride, high-purity technology, applied in the preparation of amino compounds from amines, chemical recovery, organic chemistry, etc., can solve impurities, naphthalene ethylenediamine hydrochloride solubility, sensitivity, blank value and poor reproducibility , poor quality, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
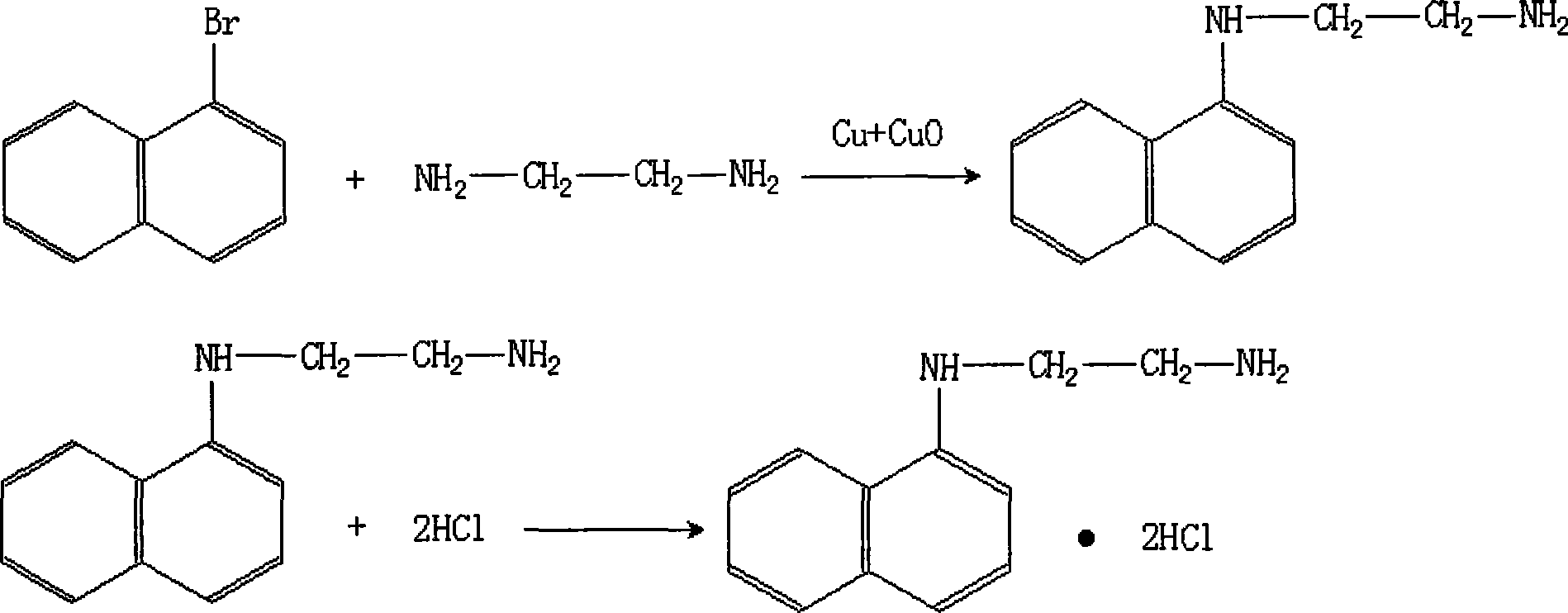
[0025] The preparation of high-purity naphthalene ethylenediamine hydrochloride:
[0026] (1). Add 69 grams of bromonaphthalene and 160 grams of ethylenediamine to a reaction kettle equipped with a mechanical stirring cooling reflux device, heat the mixture to 110°C, keep the temperature at reflux for 10 hours, and then cool to 70°C ;
[0027] (2). After mixing copper powder and copper oxide powder in a weight ratio of 100:5, take a catalytic amount of the mixture and add it to the reactor, reflux for 10 hours, and cool naturally;
[0028] (3). Excessive ethylenediamine is reclaimed by vacuum distillation;
[0029] (4). Extract the reactant with benzene, filter, and reclaim benzene by distillation under reduced pressure;
[0030] (5). Underpressure distillation obtains product naphthalene ethylenediamine;
[0031] (6). Naphthalene ethylenediamine was added to the reagent hydrochloric acid solution with a concentration of 4M, the temperature was cooled to 0°C, and the crysta...
Embodiment 2
[0034] The preparation of high-purity naphthalene ethylenediamine hydrochloride:
[0035] (1). Add 414 kg of bromonaphthalene and 960 kg of ethylenediamine to a reaction kettle equipped with a mechanical stirring cooling reflux device, heat the mixture to 130°C, keep the temperature at reflux for 6 hours, and then cool to 80°C ;
[0036] (2). After mixing copper powder and copper oxide powder in a weight ratio of 100:30, take a catalytic amount of the mixture and add it to the reactor, reflux for 6 hours, and cool naturally;
[0037] (3). Excessive ethylenediamine is reclaimed by vacuum distillation;
[0038] (4). Extract the reactant with ethanol, filter, and reclaim ethanol by distillation under reduced pressure;
[0039] (5). Underpressure distillation obtains product naphthalene ethylenediamine;
[0040] (6). Naphthalene ethylenediamine was added to the reagent hydrochloric acid solution with a concentration of 8M, cooled to 5° C. with ice, and crystallized by filtratio...
Embodiment 3
[0043] The preparation of high-purity naphthalene ethylenediamine hydrochloride:
[0044] (1). Add 207 kg of bromonaphthalene and 480 kg of ethylenediamine to a reaction kettle equipped with a mechanical stirring cooling reflux device, heat the mixture to 120°C, keep the temperature at reflux for 8 hours, and then cool to 75°C ;
[0045] (2). After mixing copper powder and copper oxide powder in a weight ratio of 100:20, take a catalytic amount of the mixture and add it to the reactor, reflux for 8 hours, and cool naturally;
[0046] (3). Excessive ethylenediamine is reclaimed by vacuum distillation;
[0047] (4). Extract the reactant with benzene, filter, and reclaim benzene by distillation under reduced pressure;
[0048] (5). Underpressure distillation obtains product naphthalene ethylenediamine;
[0049] (6). Add naphthalene ethylenediamine to the reagent hydrochloric acid solution with a concentration of 6M, cool to 4° C. with ice, and filter to crystallize;
[0050] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com