Chemiluminescence ELISA detection kit of furadantin
A chemiluminescent enzyme, nitrofurantoin technology, applied in chemiluminescence/bioluminescence, analysis by chemical reaction of materials, measurement devices, etc., can solve the problems of cumbersome derivatization, expensive and bulky instruments that are difficult to carry, and time-consuming sample processing processes. , to achieve the effect of simple sample pretreatment, low detection limit and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 11
[0030] Example 11-Derivatization of aminohydantoin (AHD), preparation of immunogen, coating antigen, and antibody
[0031] (1) Derivatization of AHD
[0032] Dissolve 75mg (0.5mmol) of 3-carboxybenzaldehyde (CBA) in 5mL of methanol to obtain liquid A. Dissolve 76mg of AHD (0.5mmol) in 15mL of methanol to obtain liquid B. Mix liquids A and B, stir, and reflux at 65°C. Tracked by TLC, the reaction is completed in about 18 hours. After rotary evaporation to dryness, about 20 mL of ethanol was added to wash and filter with suction. The AHD derivative 1-(4-carboxybenzylidene)-aminohydantoin (CPAHD) is obtained.
[0033] (2) Preparation of immunogen
[0034] The mixed acid anhydride method prepares nitrofurantoin and its metabolite (AHD) immunogen CPAHD-cBSA steps as follows:
[0035] Slowly drop 18 mg (300 μmol) of ethylenediamine (EDA) into 20 mL of ice-cold (0.01M pH7.4) phosphate buffer saline PBS, stir at 4°C, add concentrated hydrochloric acid dropwise to the solution, an...
Embodiment 2
[0042] Embodiment 2 The establishment of chemiluminescent enzyme-linked immunosorbent assay (CL-ELISA)
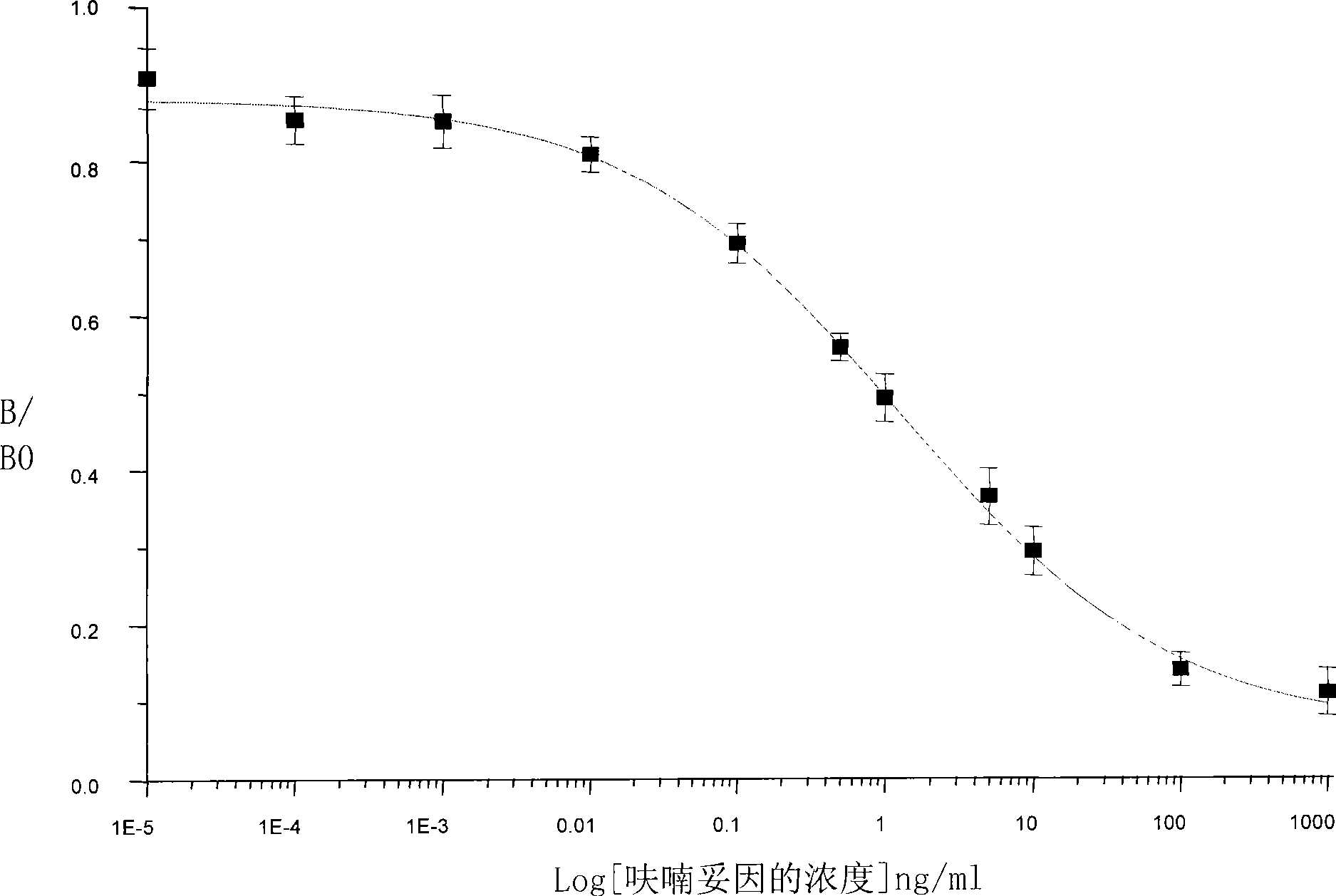
[0043] 2.1 Optimal concentration of antibody and coated antigen (square matrix)
[0044] Use 100μL / well in the longitudinal direction of the microplate plate, and the concentration gradient is 80.0μg / mL, 40.0μg / mL, 20.0μg / mL, 10.0μg / mL, 5.0μg / mL, 2.5μg / mL, 1.25μg / mL, and 0.625 Coat with μg / mL coating antigen solution, place overnight at 4°C, wash the plate three times with 280 μL / well washing solution, then block with 250 μL / well blocking solution, place at room temperature for 2.5 hours, wash three times; add 100 μL / well horizontally, Dilute the antibody solution at 1:100, 1:200~1:51200, place at room temperature for 2 hours, wash three times; add 100 μL / well of 1:1000 horseradish peroxidase-labeled goat anti-rabbit antibody, place at room temperature Wash three times for 1 hour; add 100 μL / well of luminescence solution, and measure the luminescence value.
[0045] Speci...
Embodiment 3
[0065] Embodiment 3, the assembly of the chemiluminescent ELISA kit for detection of nitrofurantoin of the present invention
[0066] (1) The composition of the chemiluminescent ELISA kit for detecting nitrofurantoin
[0067] a. A solid phase carrier (milky white opaque polystyrene 96-well chemiluminescent microplate plate) coated with antigen (conjugate of AHD and carrier protein);
[0068] b, nitrofurantoin antibody working solution (volume ratio concentration is 1:5000);
[0069] c. 6 bottles of nitrofurantoin standard solution, the concentrations are 0.1ng / mL, 0.5ng / mL, 1ng / mL, 5ng / mL and 10ng / mL;
[0070] d. Horseradish peroxidase-labeled goat anti-rabbit IgG antibody working solution (working concentration is 1:1000);
[0071] e Concentrated phosphate buffer solution: 80g of NaCl per liter, KH 2 PO 4 2.0g, Na 2 HPO 4 .12H 2 o 2 29.0g, KCl2.0g aqueous solution.
[0072] f Concentrated washing solution: a concentrated phosphate buffer solution of pH 7.5 and 0.1 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| coefficient of variation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com