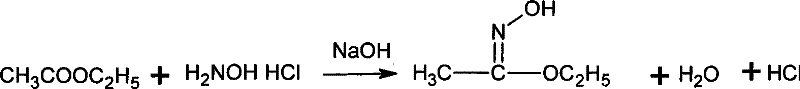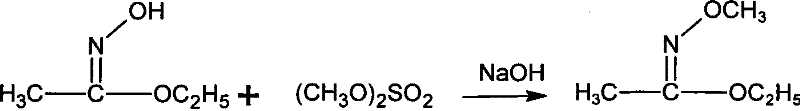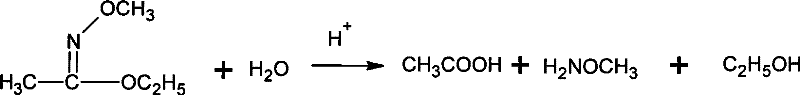Method for synthesizing methoxamine hydrochloride
A technology of methoxyamine hydrochloride and hydroxylamine hydrochloride is applied in the field of compound synthesis to achieve the effects of good atom economy, reduction of side reactions, and improvement of product yield and quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Add 10.6g (0.12mol) ethyl acetate and 7.0g (0.10mol) hydroxylamine hydrochloride into a 100mL flask equipped with electric stirring, thermometer and condenser, cool to 10℃, and start adding 40g NaOH with a mass fraction of 20% dropwise. (0.2mol) aqueous solution, drip for 1h, and react at 15°C for 0.5h after dripping. Then, 15.1g (0.12mol) of dimethyl sulfate was added dropwise at this temperature, and then the temperature was raised to 70°C for 4h. Cool to 10°C, add 200 mL of cold water, then extract three times with 100 mL of chloroform, and combine the extracts. The solvent was evaporated under reduced pressure (0.05MPa) at 30°C, the distillation residue was added to 17g of hydrochloric acid with a mass fraction of 15%, the temperature was raised to 65°C, and the temperature was kept for 0.5h. Then cool to 10°C, add dropwise 30% sodium hydroxide solution at no higher than this temperature to adjust the pH to 11, and then use heating distillation to collect the distil...
Embodiment 2
[0044] The amount of ethyl acetate was changed to 0.14 mol, and the others were the same as in Example 1. The result was 7.5 g of methoxyamine hydrochloride, the yield was 89.8%, and the product mole fraction was 99.2%.
Embodiment 3
[0046] For oximation, 24 g of 25% NaOH (0.15 mol) solution was added dropwise, and the others were the same as in Example 1. The result was 7.2 g of methoxyamine hydrochloride, the yield was 86.2%, and the product mole fraction was 98.6%. .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com