Method for aggregating acetylcholinergic receptor for stabilizing neuromuscular junction
A technology of acetylcholine receptors and neuromuscular junctions, which is applied in the field of regulating the stability of acetylcholine receptor aggregates of neuromuscular junctions, and can solve problems such as unclear effector molecules
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0125] Example 1 Cholinergic agonist CCh can activate Calpain
[0126] The activity of Calpain is regulated by calcium ions, and cholinergic agonists and depolarization of muscle cells can cause an increase in calcium ions. The present inventors examined whether cholinergic agonists could modulate the activity of Calpain in muscle cells.
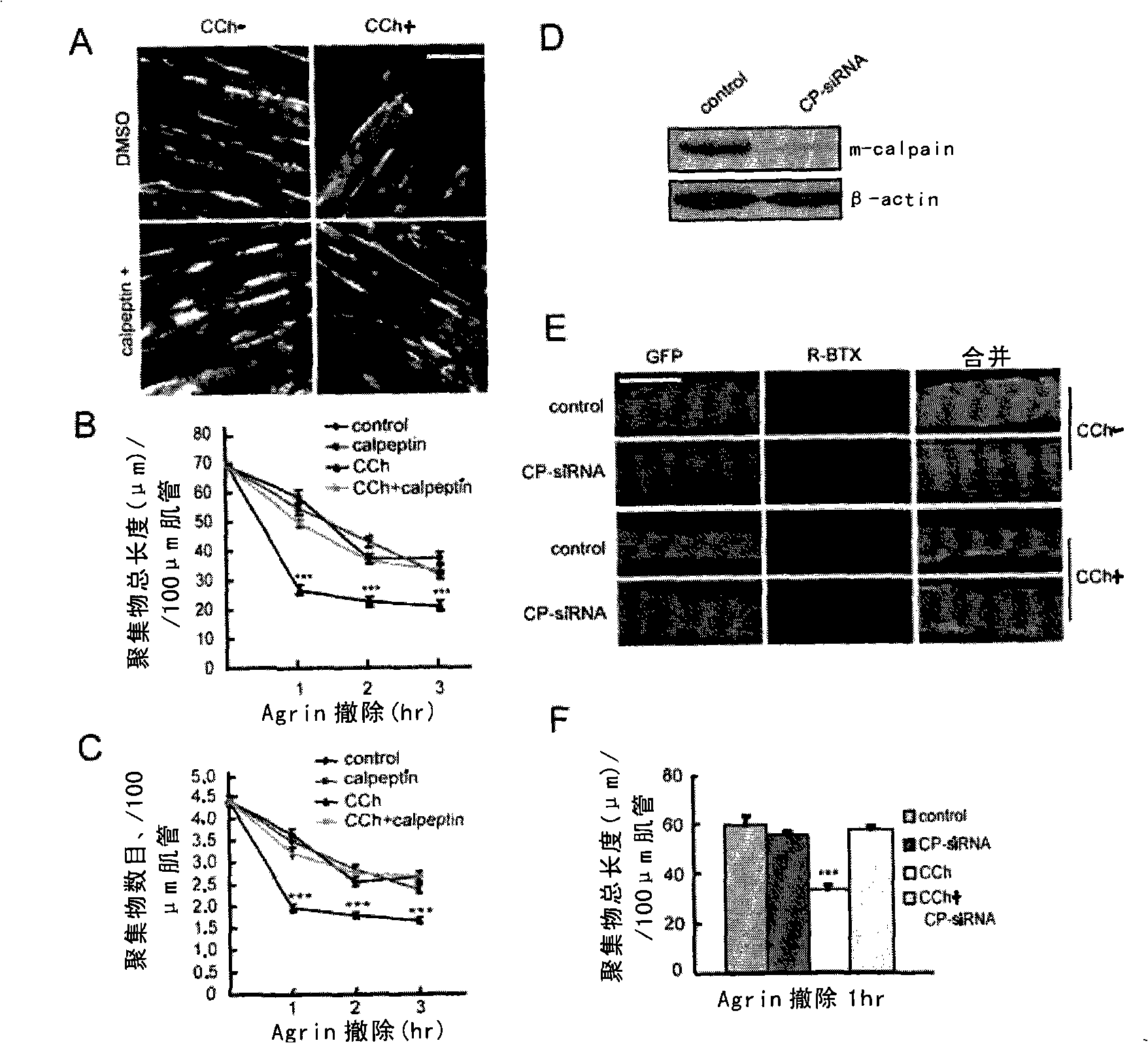
[0127] Such as figure 1 As shown in A, treatment of muscle cells with CCh, a cholinergic agonist that is not easily hydrolyzed, can not only induce calcium ion flux in muscle cells, but also increase the activity of Calpain in myotubes. Calpain activity peaked 15 min after CCh stimulation and then decayed. Calpain activation by CCh was inhibited when cells were pretreated with Calpeptin, a membrane-permeable Calpain inhibitor. These results suggest that cholinergic agonists can activate Calpain in myocytes.
[0128] Cdk5 in muscle cells can also be activated by CCh (Lin, W et al., Neurotransmitteracetylcholine negatively regulates neurom...
Embodiment 2
[0130] Example 2 Inhibiting the activity of Calpain can improve the stability of AChR aggregates
[0131] Further, the present inventors examined whether Calpain is involved in the process of cholinergic agonist-induced dissipation of AChR aggregates. Myocytes cultured in vitro were first treated with Agrin to induce AChR aggregates, then transferred to Agrin-free medium, and stimulated with CCh with or without Calpeptin treatment. The stimulation of CCh alone can promote the depolymerization of AChR aggregates, making the total length of AChR aggregates per unit length of myotubes shorter and the total number less ( figure 2 A, 2B and 2C). The reduction of AChR aggregates induced by CCh was improved in Calpeptin-treated cells ( figure 2 B and 2C), suggesting that Calpain is involved in the dissipation of AChR aggregates induced by cholinergic agonists.
[0132] In order to further understand the regulatory effect of Calpain on AChR aggregation, the inventors used the str...
Embodiment 3
[0133] Embodiment 3 Rapsyn can combine with Calpain
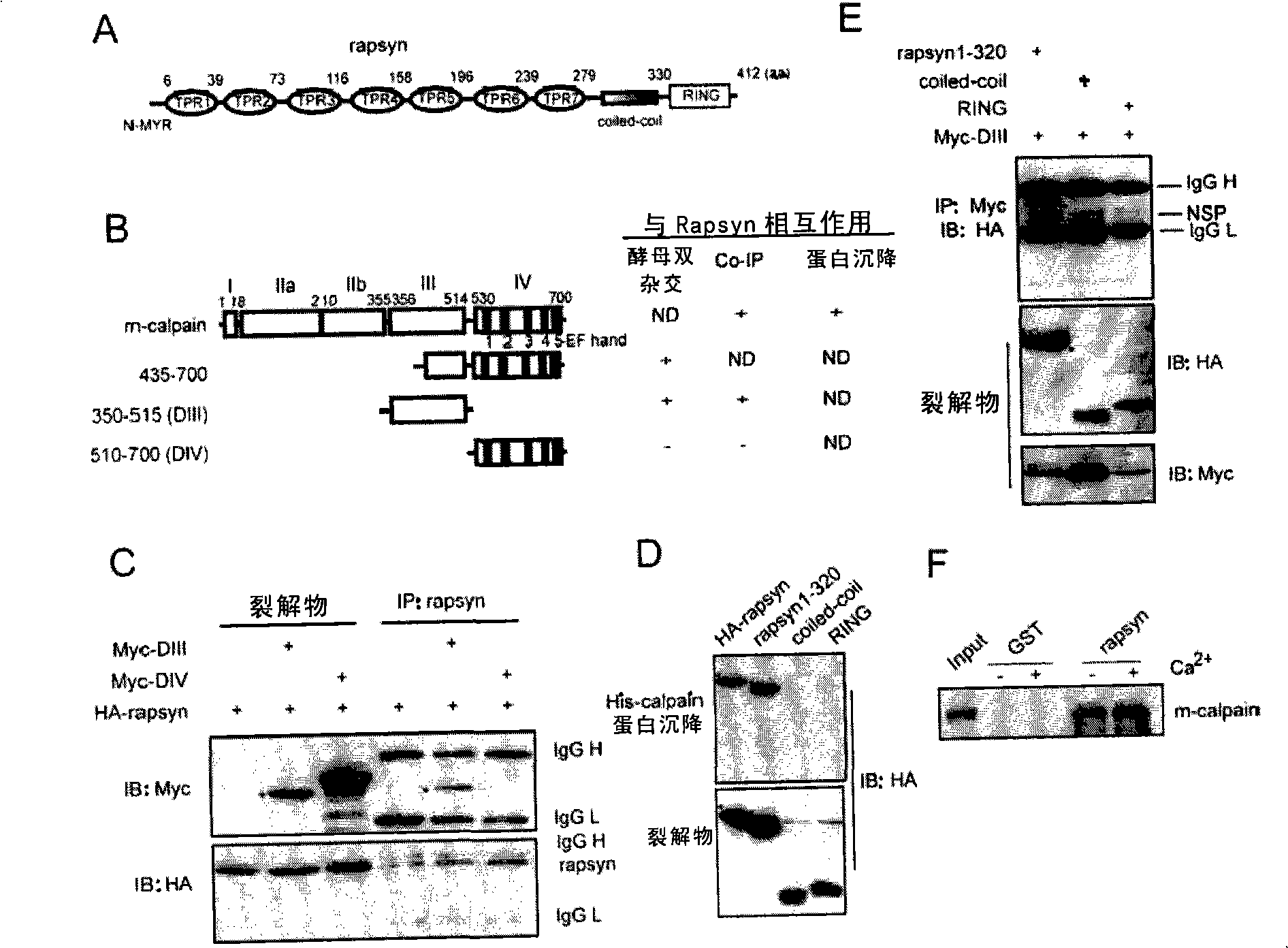
[0134] When Rapsyn was used as a bait to find its binding protein in the yeast two-hybrid system, the inventors found the large subunit of m-Calpain. M-Calpain consists of two subunits: a unique large subunit with a size of 80KD, and a small subunit with a size of 28KD shared with μ-Calpain. The large subunit itself also contains four domains: a short domain at the nitrogen end (domain I), a domain with enzymatic catalytic activity (domain II), and a domain that regulates enzymatic activity (domain III) and a calcium ion-binding domain (domain IV) comprising the EF chiral structure. The clone initially screened from yeast contained 266 amino acid sequences (equivalent to the 435-700 amino acid sequence of m-Calpain's own amino acid sequence), including domain IV and part of domain III, suggesting that the region combined with Rapsyn may be in the structure In domain III and domain IV ( image 3 B). In order to delineate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com