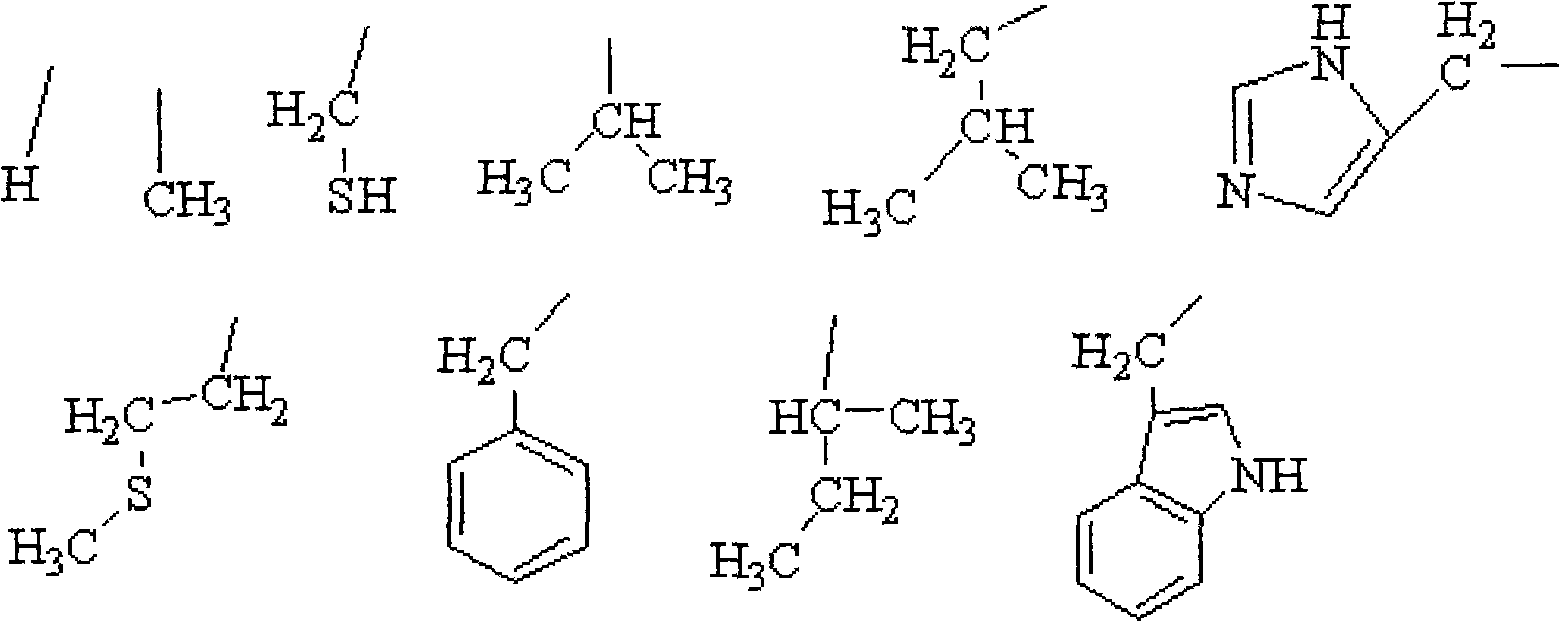
Aminoacid acidamide compounds and preparation thereof
A technology for amino acid amides and amine compounds, which is applied in the preparation of carboxylic acid amides, the preparation of organic compounds, chemical instruments and methods, etc. It can solve the problems of low reaction efficiency and stable protective groups, and achieve simple reaction methods and post-processing Easy and efficient effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
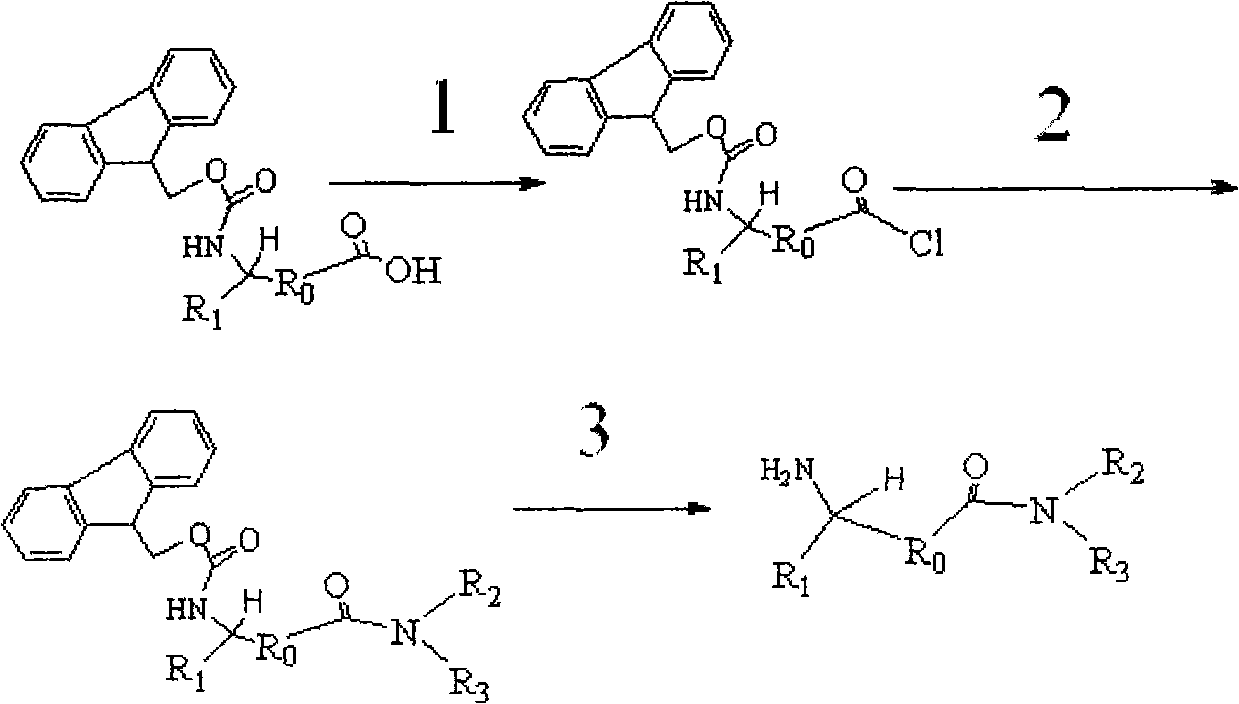
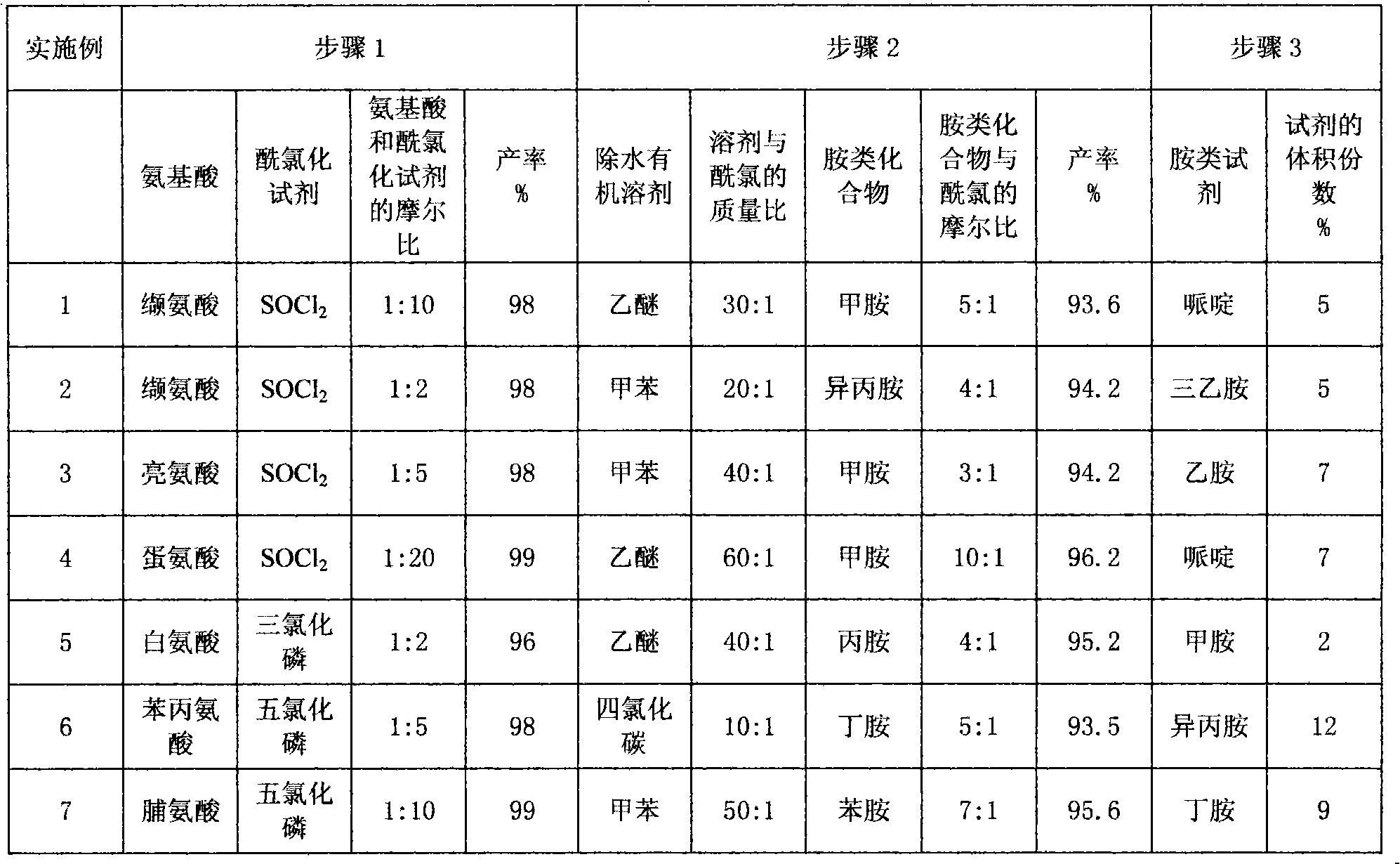
[0031] (1) Synthesis of N-Fmoc-L-valyl chloride
[0032] Dissolve 15.0g N-Fmoc-L-valine (44.2mmol) in 250ml CH 2 Cl 2 , pass into N 2 For protection, add 32.1ml SOCl dropwise 2 (442.0mmol), heated to 50°C, refluxed for 4 hours, then changed to a distillation device, and evaporated most of the CH 2 Cl 2 Afterwards, it was evaporated to dryness under reduced pressure and dried under vacuum at 60° C. for 2 h to obtain 15.5 g of N-Fmoc-L-valyl chloride as a light yellow solid with a yield of 98%.
[0033] (2) Synthesis of N-Fmoc-N'-methyl-L-valinamide
[0034] Dissolve 15.5 g (44.2 mmol) of the above solid product into 465 g of anhydrous ether, and quickly add 26.0 g of an aqueous solution of 25% to 30% methylamine (221.0 mmol) 5 times the molar weight of the above solid product after dissolution, and shake vigorously for 3 minutes , suction filtration, the solid was washed in 800ml of water, suction filtration, and then washed with 800ml of water, suction filtration, repeat...
Embodiment 2
[0038] (1) Synthesis of N-Fmoc-L-valyl chloride
[0039]Dissolve 15.0g N-Fmoc-L-valine (44.2mmol) in 250ml CH 2 Cl 2 , pass into N 2 For protection, add 32.1ml SOCl dropwise 2 (442.0mmol), heated to 50°C, refluxed for 4 hours, then changed to a distillation device, and evaporated most of the CH 2 Cl 2 Afterwards, it was evaporated to dryness under reduced pressure and dried under vacuum at 60° C. for 2 h to obtain 15.5 g of N-Fmoc-L-valyl chloride as a light yellow solid with a yield of 98%.
[0040] (2) Synthesis of N-Fmoc-N'-isopropyl-L-valinamide
[0041] 15.5 g (44.2 mmol) of the above-mentioned solid product was dissolved in 310 g of anhydrous toluene. After the dissolution was complete, 10.1 g of isopropylamine (176.8 mmol) solution of 4 times the molar weight of the above-mentioned solid product was quickly added, vigorously shaken for 4 minutes, suction filtered, and The solid was washed in 600ml of water, filtered with suction, and then washed with 600ml of wate...
Embodiment 3
[0045] (1) Synthesis of N-Fmoc-L-leucyl chloride
[0046] Dissolve 10.0g N-Fmoc-L-leucine (28.3mmol) in 75ml CH 2 Cl 2 , pass into N 2 For protection, add 20.6ml SOCl dropwise 2 (283.0mmol), heated to 50°C, and refluxed for 2 hours, then changed to a distillation device, and evaporated most of the CH 2 Cl 2 Afterwards, it was evaporated to dryness under reduced pressure and dried under vacuum at 60° C. for 2 h to obtain 10.3 g of N-Fmoc-L-leucyl chloride as a light yellow solid with a yield of 98%.
[0047] (2) Synthesis of N-Fmoc-N'-methyl-L-leucineamide
[0048] Dissolve 10.0 g (26.9 mmol) of the above solid product into 400 g of anhydrous toluene. After the dissolution is complete, quickly add 9.5 g of an aqueous methylamine solution (80.7 mmol) 3 times the molar weight of the above solid product, shake vigorously for 2 min, and filter with suction. The solid was washed in 600ml of water, filtered with suction, washed with 600ml of water again, filtered with suction, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com