Non-virus non-structural protein foot-and-mouth disease vaccine and preparation thereof
A non-structural protein, foot-and-mouth disease vaccine technology, applied in antiviral immunoglobulins, biochemical equipment and methods, antiviral agents, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
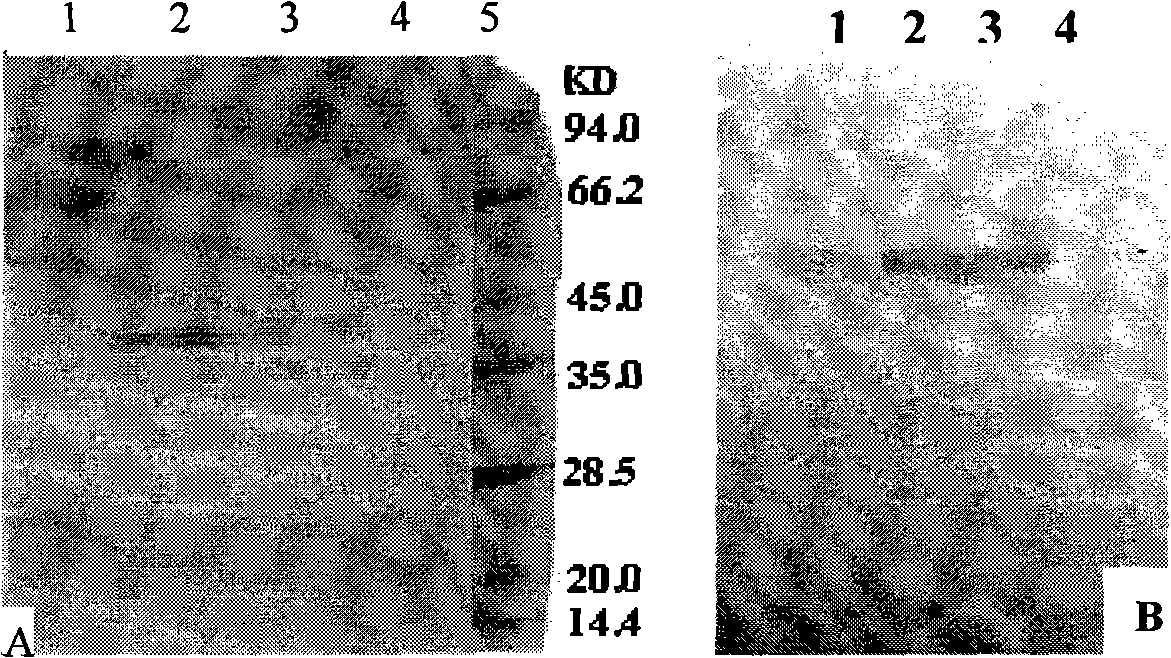
Image
Examples
Embodiment 1
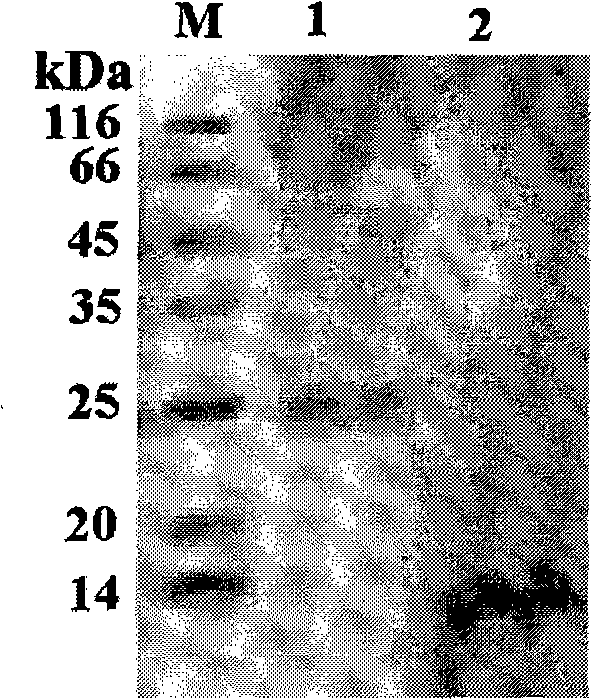
[0036] Embodiment 1, the preparation of the monoclonal antibody of foot-and-mouth disease virus nonstructural protein (2C3AB, 3A, 3B, 3ABC)
[0037] The main reagents required for the preparation of monoclonal antibodies in this example are as follows:
[0038] (1) The cell culture medium RPMI-1640 used in the hybridoma technology is a product of Sigma Company, and the fetal bovine serum is a product of Hyclone Company, both of which are stored at 4°C.
[0039] (2) Incomplete RPMI-1640 medium: 96ml of RPMI-1640 medium stock solution, 1ml of 100×L-glutamine solution, 1ml of double antibody solution, 7.5% NaHCO 3 Solution 1-2ml.
[0040] (3) Complete RPMI-1640 medium: 80 ml of incomplete RPMI-1640 medium, 15-20 ml of fetal bovine serum, used for culturing myeloma cells SP2 / 0 and hybridoma cells after strain establishment.
[0041] (4) HT medium: 98 ml of complete RPMI-1640 medium, 2 ml of 50×HT stock solution.
[0042] (5) HAT medium: 96 ml of complete RPMI-1640 medium, 2 ml ...
Embodiment 2
[0146] Embodiment 2, preparation and detection of the foot-and-mouth disease vaccine without virus non-structural protein
[0147] There are three groups of experiments in this embodiment. The first group of experiments is to use the first group of monoclonal antibodies (i.e. 3A protein monoclonal antibody (produced by the secretion of the foot-and-mouth disease virus 3A monoclonal antibody cell line FMDV 3A McAb with the deposit number of 2585) and 3B protein The monoclonal antibody (produced by the secretion of the foot-and-mouth disease virus 3B monoclonal antibody cell line FMDV 3B McAb with the preservation number 2586) removes the non-structural protein of the virus to prepare a foot-and-mouth disease vaccine without the non-structural protein of the virus, and detects it; the second group of experiments is to use The second group of monoclonal antibodies (that is, the 3ABC protein monoclonal antibody is secreted by the foot-and-mouth disease virus 3ABC monoclonal antibod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com