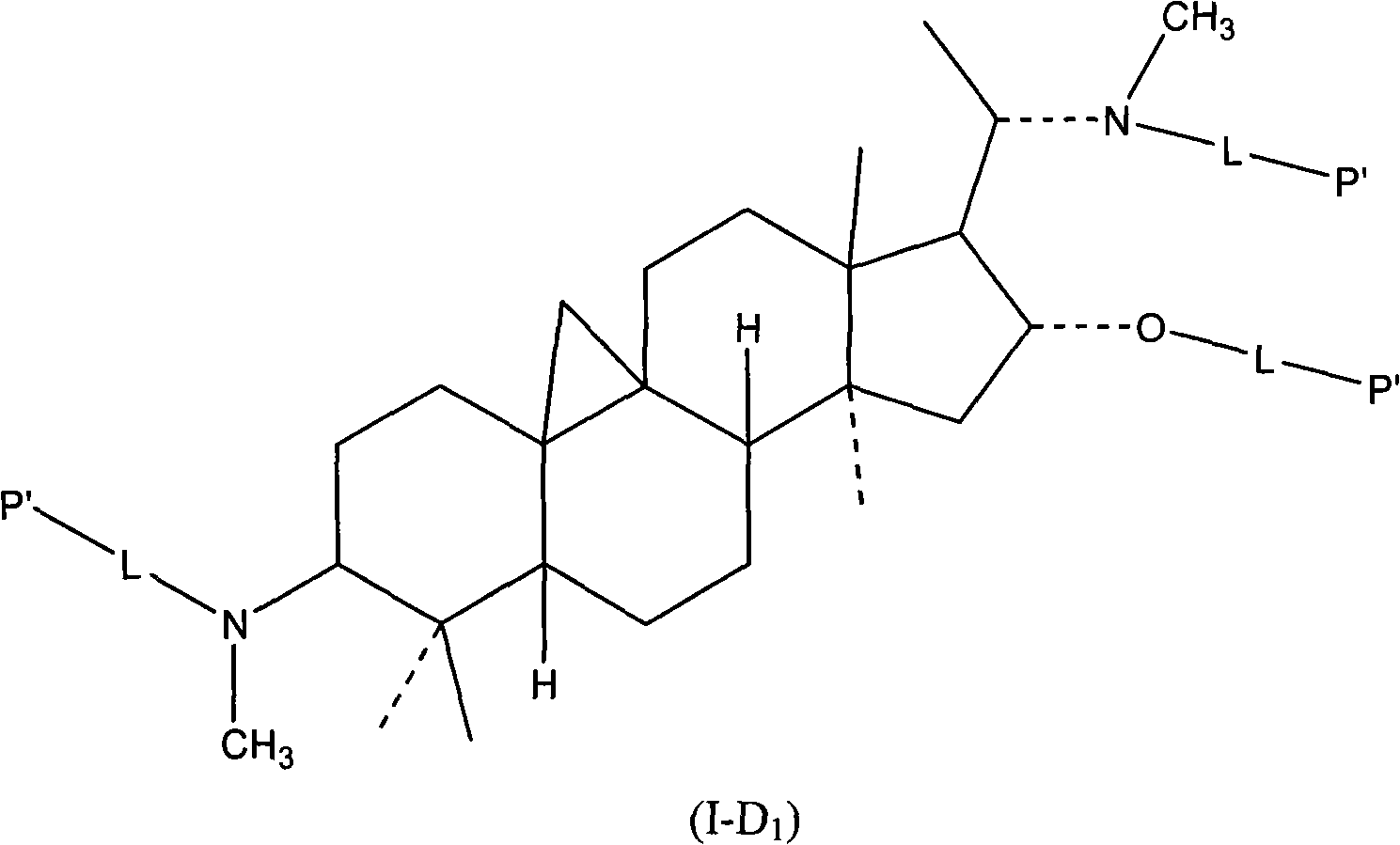
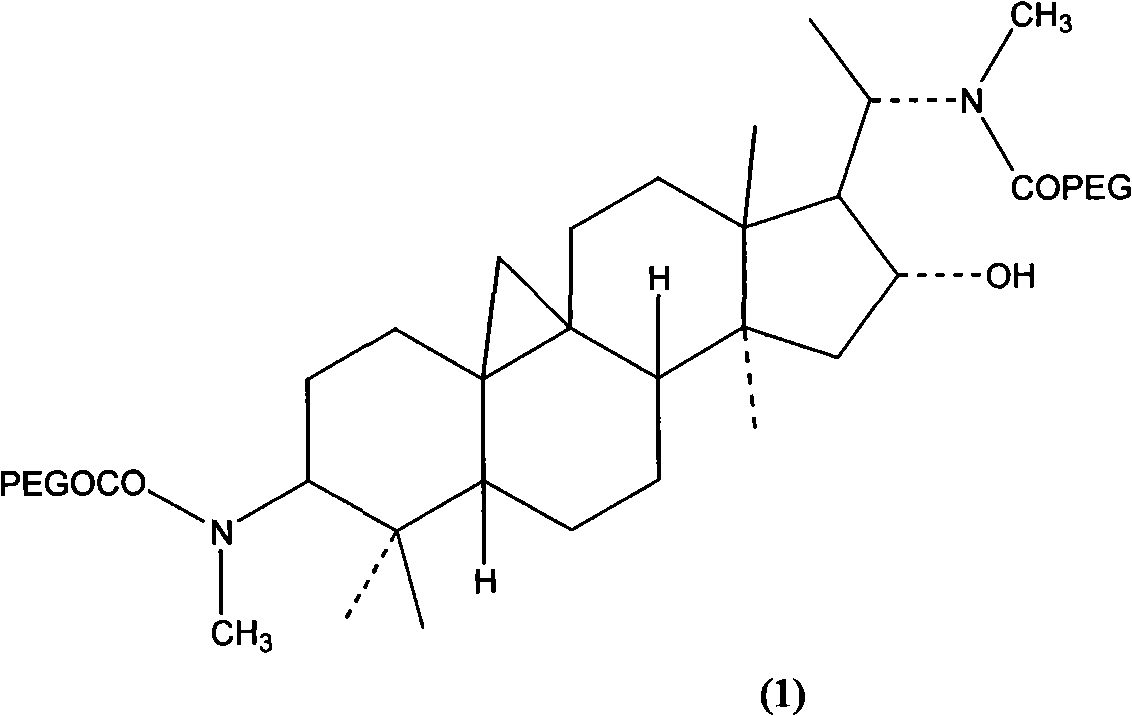
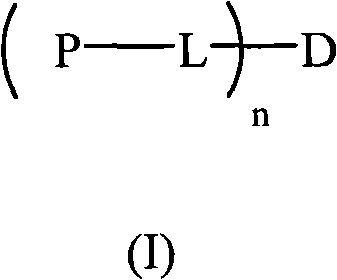Hydrophilic polymer-boxwood extract conjugate and its medicine composition
A technology of hydrophilic polymers and conjugates, which is applied in the direction of drug combinations, steroids, pharmaceutical formulations, etc., can solve the problems of poor blood, short half-life, easy crystallization, etc., to improve stability and water solubility, prolong The effect of the active cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Synthesis of amido-bonded polyethylene glycol acetic acid and cycloevergreen boxine D
[0061]
[0062] 5 grams of methoxypolyethylene glycol acetic acid (mPEG-O-CH 2 -COOH, Mw5000), 0.25 grams of Cyclophylline D (D 1 ), 0.2 g of 4-dimethylaminopyridine (DMAP) was dissolved in 50 ml of anhydrous dichloromethane, and 0.32 g of dicyclohexylcarbodiimide (DCC) was added. Stir overnight under nitrogen protection, excess solvent is removed by rotary evaporation, and 20 ml of 1,4-dioxane is added to the residue. The precipitate was removed by filtration and the filtrate was partially concentrated by rotary evaporation. 100 mL of isopropanol was added to the residue, and the product was collected by filtration and dried in vacuo. N,N'-dimethoxypolyethylene glycol acetic acid-cycloevergreen boxine D amide (1) was obtained, yield: 4.2 g (83%), melting point: 57-59°C.
Embodiment 2
[0064] Synthesis of Polyethylene Glycol Polycarboxy-Oligopeptide and Cycloevergreen Echrysene D Bonded with Amide Group
[0065]
[0066] The method is the same as in Example 1, except that methoxypolyethylene glycol acetic acid is replaced with methoxypolyethylene glycol-glutamic acid dipeptide (Mw10500). Methoxypolyethylene glycol glutamic acid dipeptide-cycloevergreen boxine D amide (2) was obtained, yield: 0.9 g (90%), melting point: 58-59°C.
Embodiment 3
[0068] Synthesis of Amide-Bonded Polyglutamic Acid and Cycloevergreen Box D
[0069]
[0070] The method is the same as in Example 1, except that methoxypolyethylene glycol acetic acid is replaced by polyglutamic acid (Mw5000). Polyglutamic acid-cycloevergreen boxine D amide (3) was obtained, yield: 0.9 g (90%).
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com