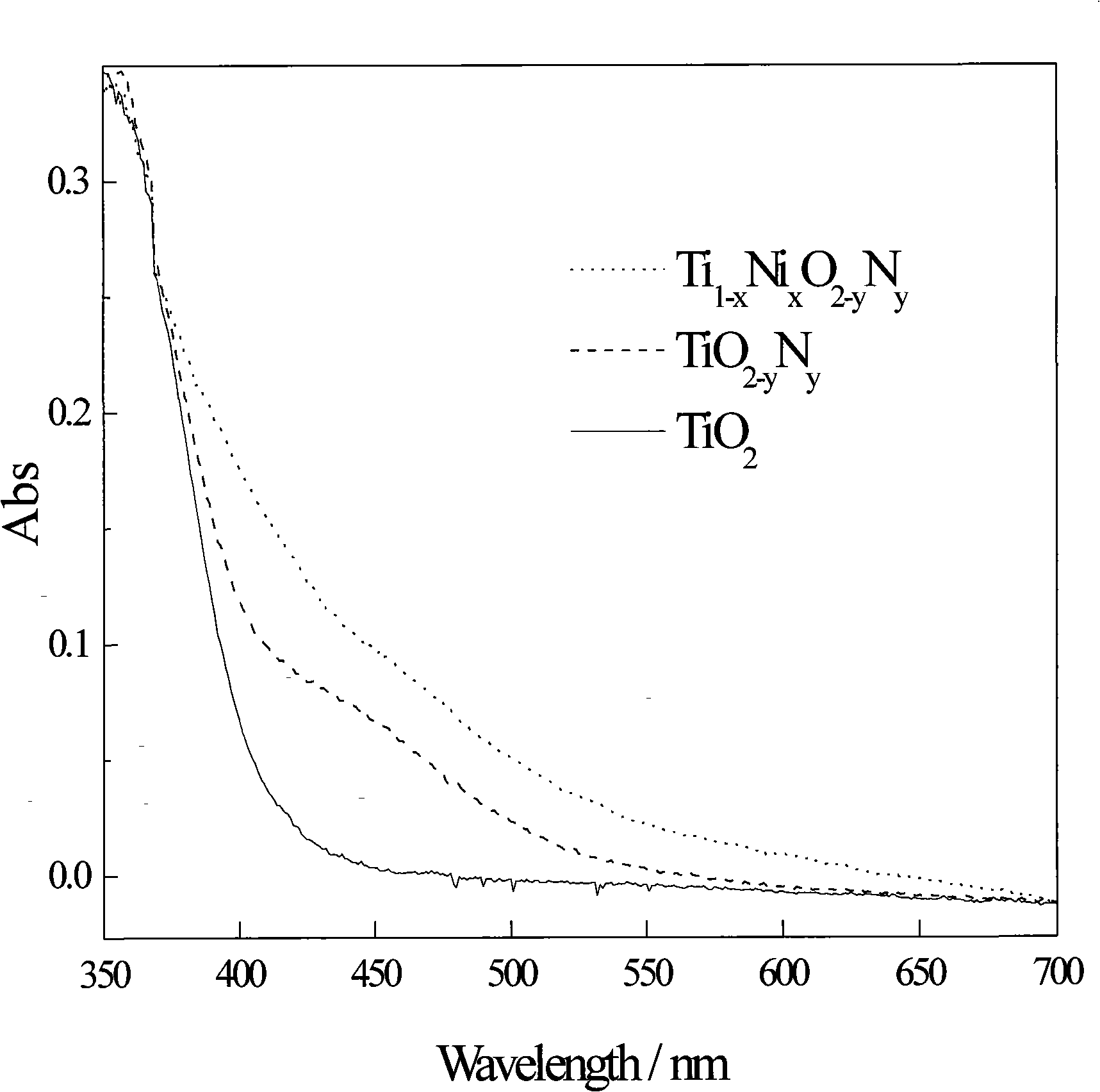
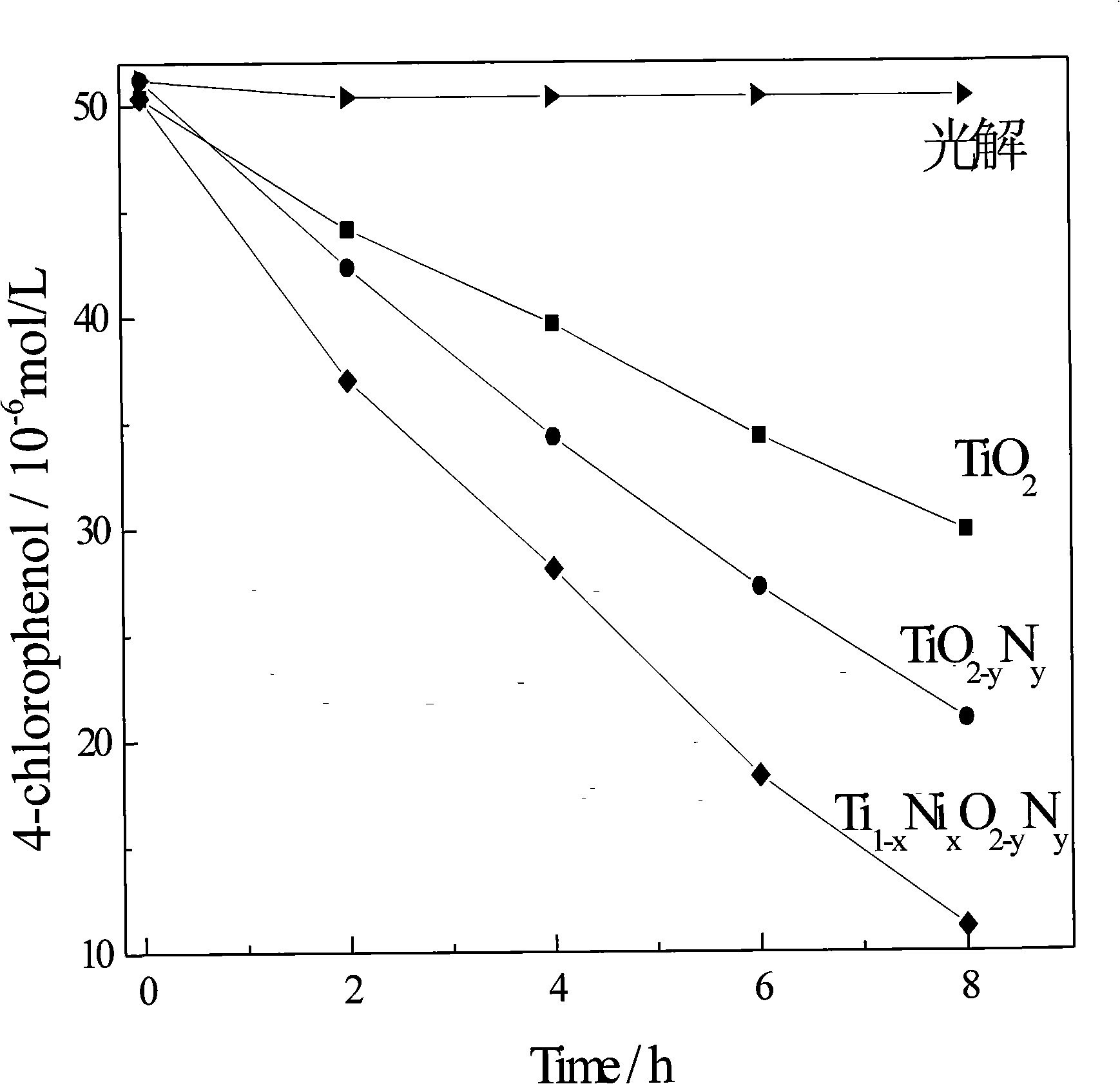
Method for preparing high efficiency metallic, non-metallic ion co-doped nano-TiO2 visible-light responsive photocatalyst
A catalyst and non-metallic technology, applied in the field of photocatalysis, to achieve the effect of simple preparation process, low equipment requirements, and enhanced inhibition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0022] ②Pure TiO 2 Catalyst preparation
[0023] Preparation steps and Ti 1-x A x O 2-y B y Similar, except that no metal salt is added, and high-purity water (18.2MΩcm -1 ) Or the corresponding alcohol instead of the solution of the compound containing the non-metallic element for dropwise addition.
[0024] 3. When preparing the above sol, the amount of various reactants added is:
[0025] The molar ratio of metal salt to titanate is 1:1000-1:2
[0026] The volume ratio of diluent to titanate is 1:10-20:1
[0027] The volume ratio of acid catalyst to titanate is 1:30-1:2
[0028] The molar ratio of the compound containing non-metal elements to the titanate is 1:30-10:1
[0029] The titanate is any one or a mixture of tetrabutyl titanate, isopropyl titanate, propyl titanate or ethyl titanate.
[0030] The diluent is any one or a mixture of anhydrous ethanol, anhydrous methanol, propanol or isopropanol.
[0031] The acid catalyst is any one or a mixture of hydrochloric acid, sulfu...
Embodiment 1
[0037] 1. Nitrogen and nickel ions co-doped visible light catalyst (Ti 1-x Ni x O 2-y N y ) Preparation
[0038] At room temperature, 628.4mg of NiCl 2 ·5H 2 O was dissolved in 40ml of absolute ethanol, stirred until completely dissolved, 15ml of tetrabutyl titanate was slowly dropped into the reaction system, stirred for 0.5h, and then 4.16ml of concentrated hydrochloric acid was added dropwise (to control the sol-gel The hydrolysis process of the system makes the polycondensation reaction complete), stirring, and after 12 hours of standing and aging, 3ml of ammonia water is slowly dropped during the stirring process, and a large amount of white precipitate appears. After stirring for 3 hours at room temperature, the system became a white gel. The gel was dried at 100°C, ground into powder, and sintered at 450°C for 2 hours to obtain a visible light catalyst co-doped with nitrogen and nickel ions ( Ti 1-x Ni x O 2-y N y )sample.
[0039] 2. TiO 2-y N y Catalyst preparation
[004...
Embodiment 2
[0044] 1. Visible light catalyst co-doped with boron and zinc ions (Ti 1-x Zn x O 2-y B y ) Preparation
[0045] Add 1.098g zinc acetate (molecular formula Zn(CH3COO) 2 ·2H 2 O) Dissolve in 25ml of absolute ethanol, stir to dissolve, slowly drop 15ml of tetrabutyl titanate into the reaction system, stir for 0.5h, and then add 4.16ml of concentrated hydrochloric acid dropwise (control the sol-gel system The process of hydrolysis, the polycondensation reaction is complete), stir, stand and age for 2 hours, slowly drop in the ethanol solution of boric acid (0.307g of boric acid dissolved in 25ml of absolute ethanol) during the stirring process, and continue to stir at room temperature When the system becomes a gel, the gel is dried at 100°C, ground into powder, and sintered at 450°C for 2 hours to obtain a visible light catalyst co-doped with boron and zinc ions (Ti 1-x Zn x O 2-y B y )sample.
[0046] 2. TiO 2-y B y Catalyst preparation
[0047] Preparation steps and Ti 1-x Zn x O 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com