Asymmetric aromatic diamine having naphthalenone binaphthyl structure, preparation and use thereof
A technology of xinthaphthylone binaphthyl and aromatic diamine, which is applied in the field of aromatic diamine compound and its preparation and application, can solve the loss of polyimide, only between 200-300°C, polyimide is insoluble, etc. problems, to achieve good film-forming properties, excellent mechanical properties, and high light transmittance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
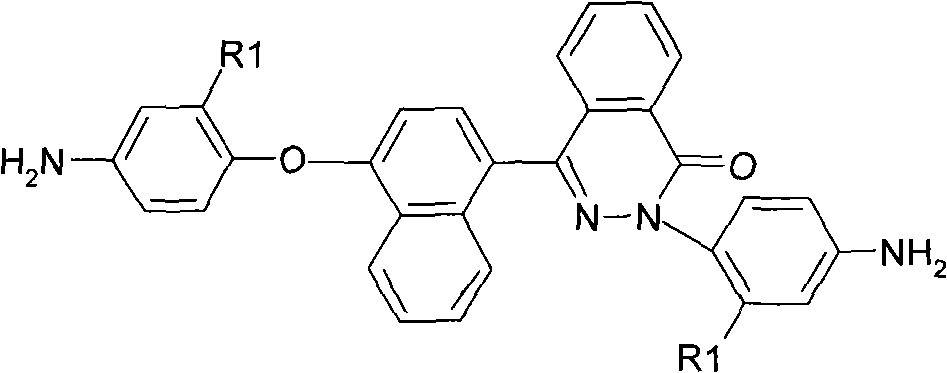
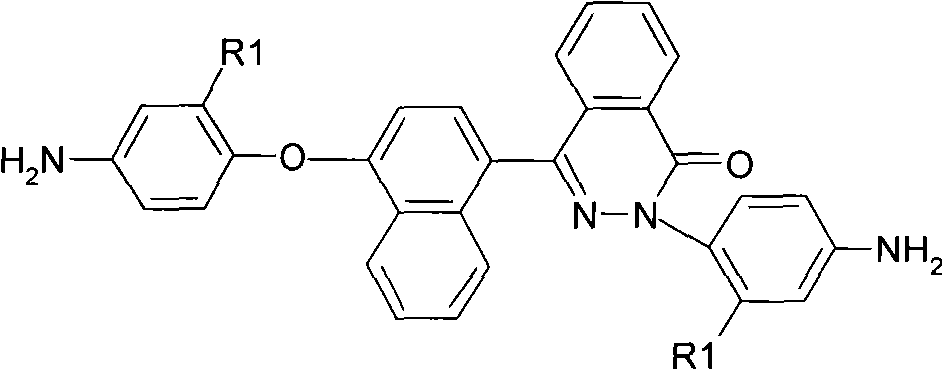
[0036] Preparation of 2-(4-aminophenyl)-4-[4-(4-aminophenoxy)-naphthyl]-phthalazin-1-one:
[0037] ①Under nitrogen protection, add 14.4g (0.05mol) 4-(4-hydroxynaphthyl)-2,3-naphthyridine-1-one and 15.8g (0.1mol) p-chloronitrobenzene to 500ml Add 14.5g (0.105mol) of potassium carbonate, 100ml of N,N-dimethylacetamide and 40ml of toluene to a three-necked flask, dehydrate using a water separator, and react azeotropically at 140-150°C for 3-6 hours until dehydration Completely, then the toluene was evaporated by heating up and the reaction was completed after reflux reaction at about 168°C for 3 hours. Crystallization gave a dark green dinitro compound: 2-(4-nitrophenyl)-4-[4-(4-nitrophenoxy)-naphthyl]-phthalazin-1-one, yield About 80-90%, melting point 239-241 ℃. FT-IR(KBr)v / cm -1 : 1675 (C=O), 1519, 1345 (-NO 2 ), 1245(C-O-C). 1H NMR (DMSO-d 6 , 400MHz) δ: 8.51(d, 1H), 8.38(d, 2H), 8.29(d, 2H), 8.06~8.10(m, 3H), 7.98(t, 1H), 7.89(m, 2H), 7.82 (d, 2H), 7.64 (t, 1H), 7.57 ...
Embodiment 2
[0040] 2-(4-amino-2-trifluoromethylphenyl)-4-[4-(4-amino-2-trifluoromethylphenoxy)-naphthyl]-naphthyridine-1 - Preparation of ketones:
[0041] ①Under nitrogen protection, mix 14.4g (0.05mol) 4-(4-hydroxynaphthyl)-2,3-naphthyridine-1-one and 22.6g (0.1mol) 2-chloro-5-nitro Add benzotrifluoride to a 500ml three-necked flask, then add 14.5g (0.105mol) potassium carbonate and 120ml N,N-dimethylformamide respectively, react at 100-150°C for 10-20h, and pour the product into 1000ml methanol In / water (1:1), fully stir the precipitate and filter, and wash the product repeatedly with hot water, and further separate and purify it by column chromatography to obtain a white fluorine-containing dinitro compound: 2-(4-nitro-2-trifluoroform phenyl)-4-[4-(4-nitro-2-trifluoromethylphenoxy)-naphthyl]-phthalazin-1-one, the yield is about 80-90%, and the melting point is 112 ~114°C. 1681 (C=O), 1536, 1333 (-NO 2 ), 1264(C-O-C), 1152(CF 3 ). 1 H NMR (DMSO-d 6 , 400MHz) δ: 8.75(d, 1H), 8.65...
Embodiment 3
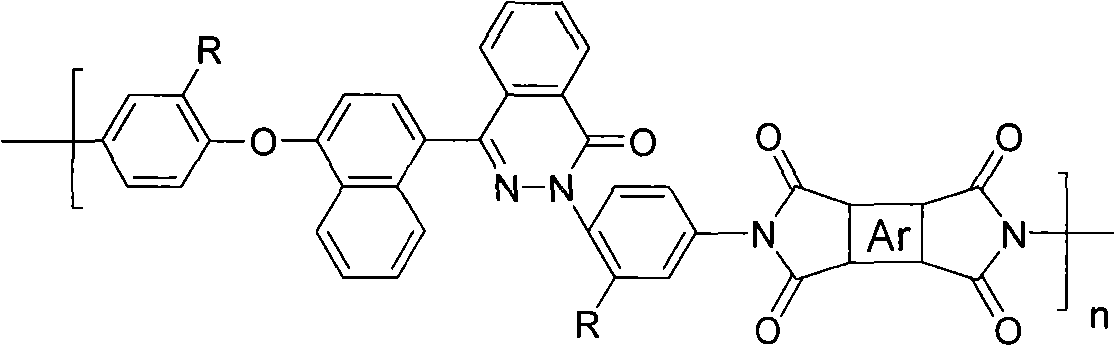
[0044] Preparation of polyimide:
[0045] Add 1.5mmol asymmetric diamine and 1.5mmol pyromellitic dianhydride monomer containing xinhone-binaphthyl structure respectively in the 50ml three-necked round-bottomed flask that is dried and ventilated with nitrogen, then add 15ml m-cresol solvent (system solid The content is 5-20%), then add about 0.3ml of isoquinoline as a catalyst, raise the temperature of the reaction system to 100-120°C for 2-3 hours, then raise the temperature to 190-200°C for about 15 hours, cool to 120°C Pour the polymer solution into methanol, collect the precipitate by filtration, wash it twice with boiling water, and dry it in vacuum at 150°C to obtain polyimide.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com