Pigment dispersion composition, optical solidifying composition, color filter and manufacturing method thereof
A pigment dispersion and photocurable technology, applied in the fields of filters, optics, organic dyes, etc., can solve the problems of reduced alkali solubility, difficult introduction, and insufficient pigment dispersion and stability, and achieve high pigment dispersion. good stability, high dispersion stability and good color properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
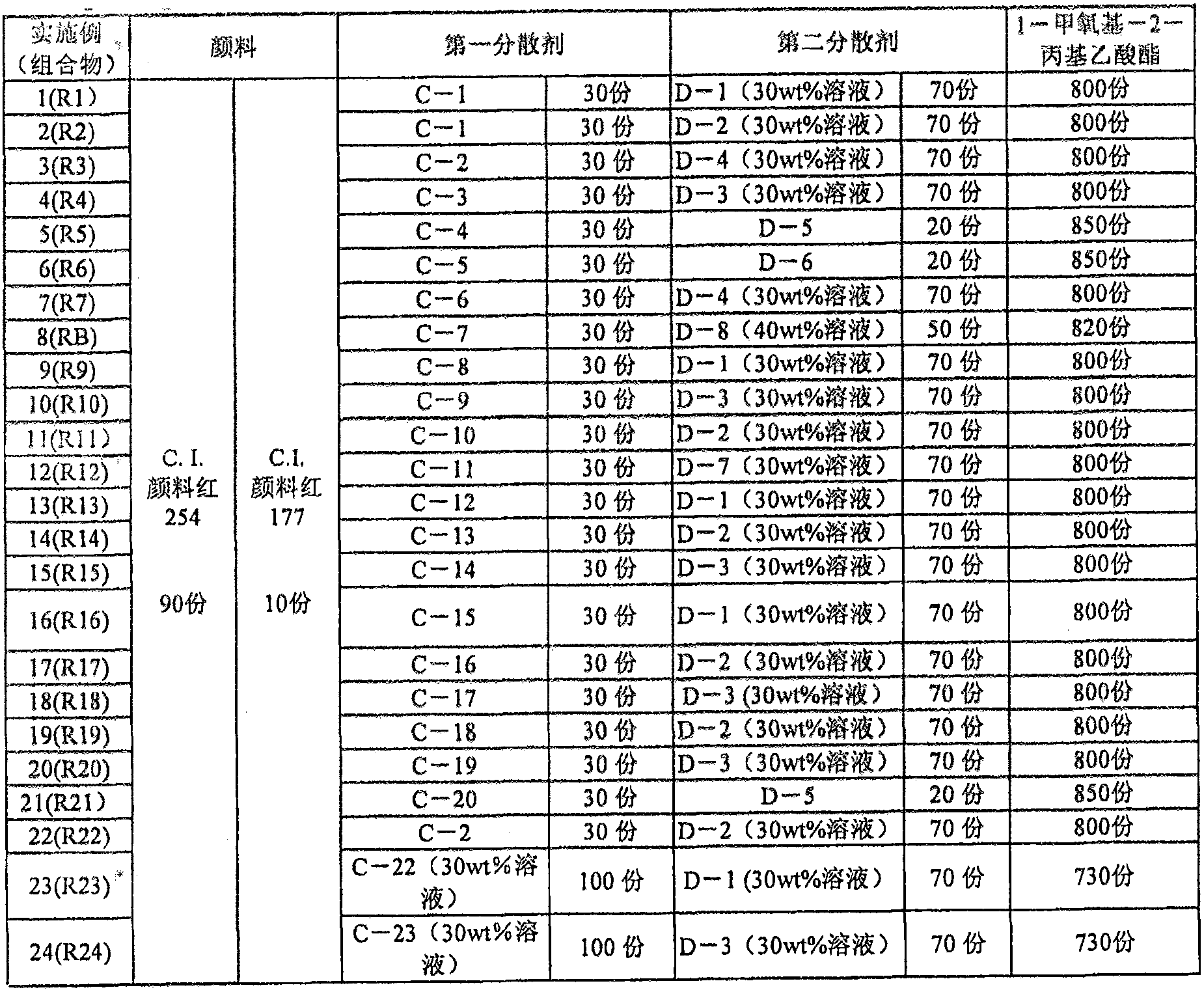
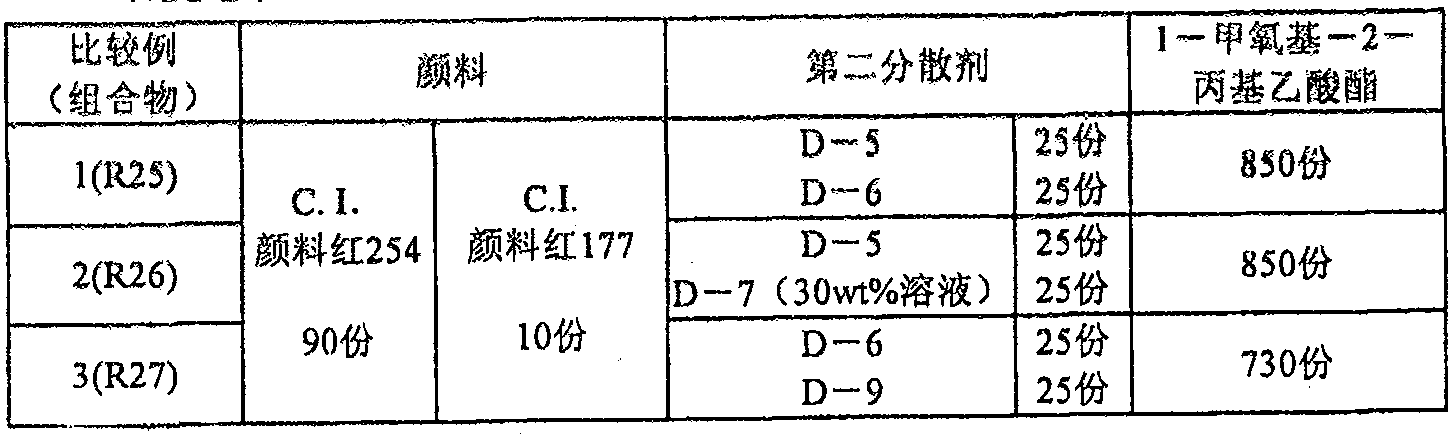
[0484] Although the present invention will be described in more detail below using examples, it is not limited to the following examples. However, unless otherwise specified, "%" and "part" are mass standards.
[0485]
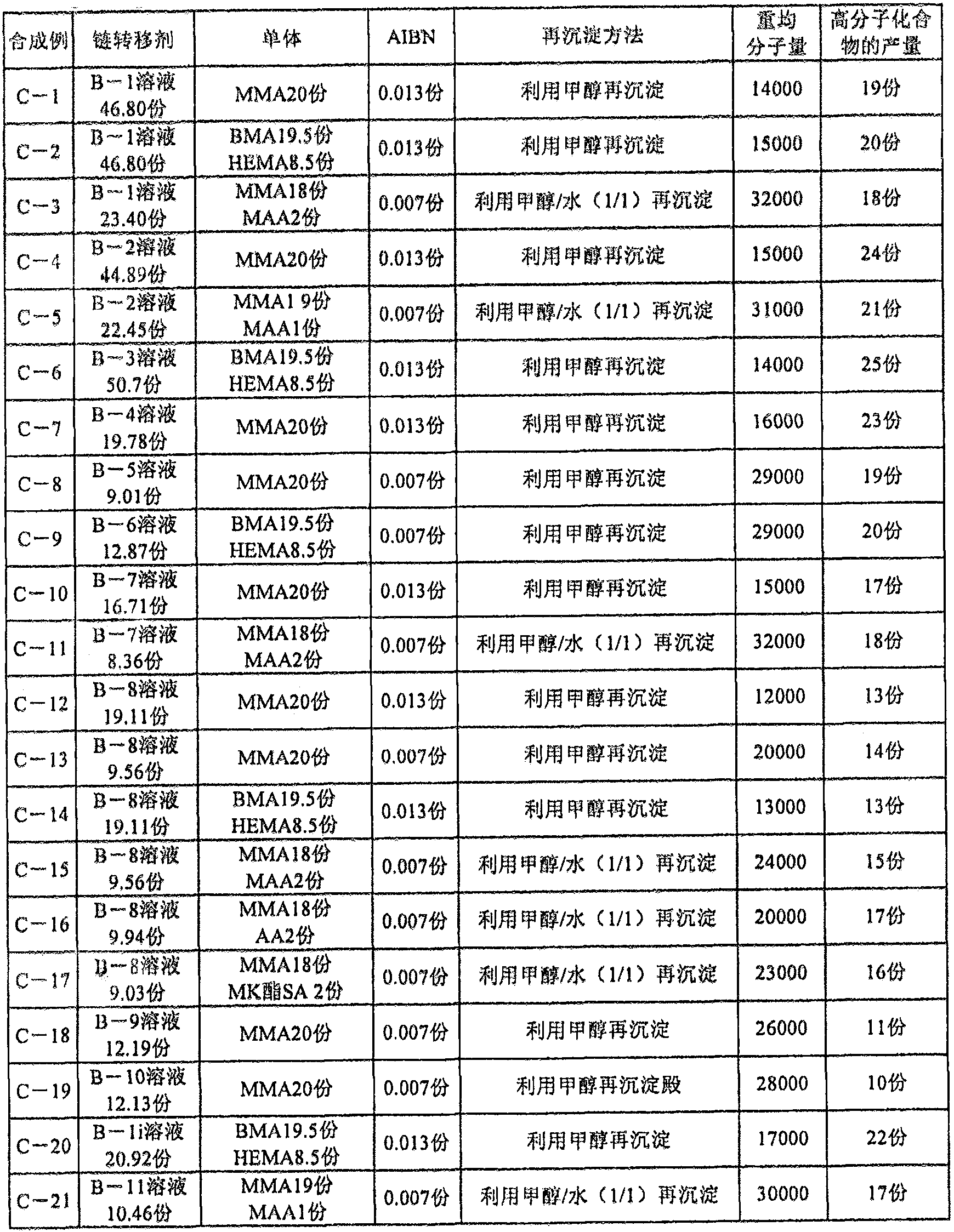
[0486] Chain transfer agents B-1 to B-12 (thiol compounds represented by the general formula (3) already described) were synthesized as shown below.
Synthetic example B-1
[0488] In 93.60 parts of dimethylformamide, 7.83 parts of dipentaerythritol hexa(3-mercaptopropionate) [DPMP; manufactured by Sakai Chemical Industry Co., Ltd., the following compound (33)] and 15.57 parts having adsorption sites and having carbon - A carbon double bond compound (the following compound (A-1)), heated to 70°C under a nitrogen stream. 0.06 parts of 2,2'-azobis(2,4-dimethylvaleronitrile) [V-65, manufactured by Wako Pure Chemical Industries, Ltd.] was added thereto, followed by heating for 3 hours. Furthermore, 0.06 part of V-65 was added, and it was made to react at 70 degreeC under nitrogen flow for 3 hours. By cooling to room temperature, a 20% solution of the thiol compound (chain transfer agent B-1) in the present invention shown below was obtained.
[0489]
Synthetic example B-2
[0491] In the above Synthesis Example B-1, 15.57 parts of the compound (A-1) having an adsorption site and a carbon-carbon double bond and 93.60 parts of dimethylformamide were changed to 14.61 parts of a compound (A-1) having an adsorption site and a carbon-carbon double bond. Bonded compound (A-2) and 89.78 parts of dimethylformamide were carried out in the same manner as above-mentioned Synthesis Example B-1 to obtain the thiol compound (chain transfer agent B- 2) A 20% solution.
[0492]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com