An uro-genital condition treatment system
A reproductive system, disease technology, applied in the field of treatment of urogenital system diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment I
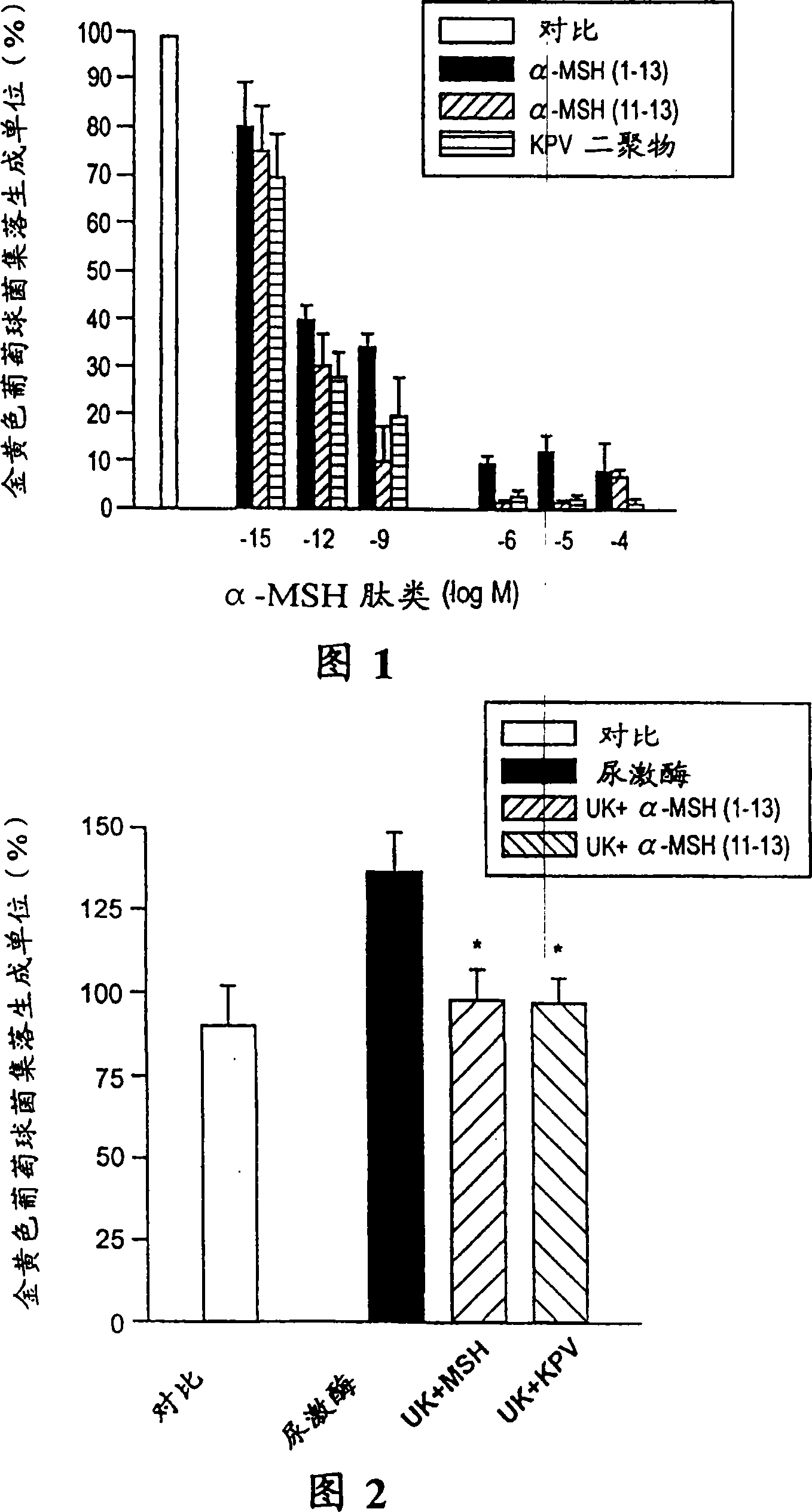
[0042] The purpose of this example is to clarify the antimicrobial properties of α-MSH and / or its derivatives. Here, Staphylococcus aureus is taken as an example. The Staphylococcus aureus culture (ATCC 29213) was provided by the Department of Microbiology of Ospedale Maggiore di Milano. Staphylococcus aureus (in Hank's balanced salt solution 1×10 6 / Ml) In the presence or absence of α-MSH (1-13) (serial number 4), α-MSH (11-13) (serial number 1) or KPV dimer, culture at 37°C 2 Hour, the peptide concentration is 10 -15 -10 -4 M. The cells were then rinsed in cold distilled water and diluted with HBSS to 100 organisms / ml. Spread a microliter aliquot on a plasma agar plate and incubate at 37°C for 24 hours. Then the viability of the microorganisms was evaluated based on the colonies formed. In another experiment, the bacteria concentration was 10 5 Add different peptides and 500 units of urokinase (growth promoter of Staphylococcus aureus) to 100 ml of culture solution, and shake cu...
Embodiment II
[0045] The purpose of this example is to clarify the antifungal properties of α-MSH and / or its derivatives. The experimental strain selected here is Candida albicans.
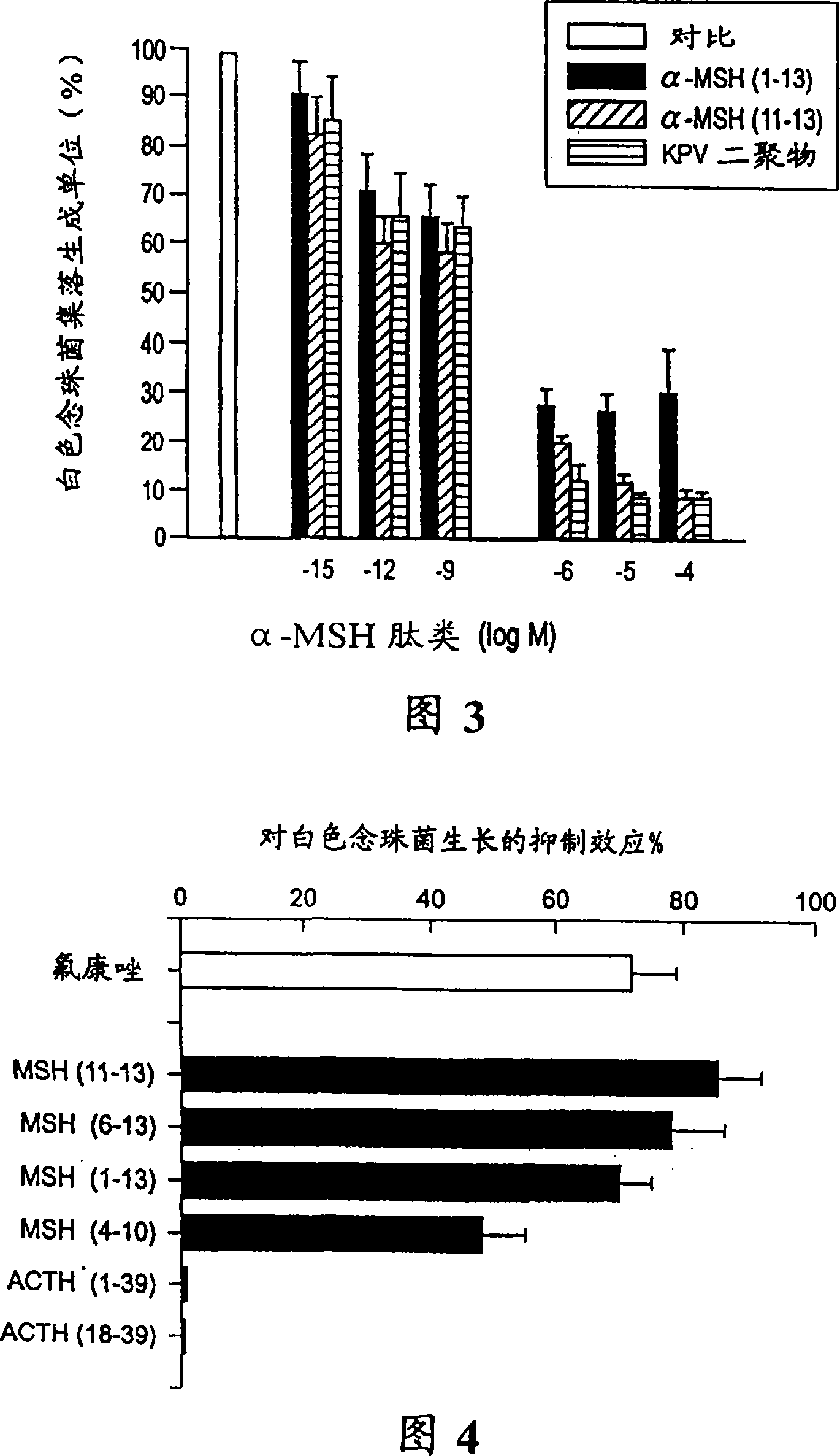
[0046] The clinical isolate of Candida albicans was provided by the Department of Microbiology of Ospedale Maggiore di Milano. Cultivation of Candida albicans was carried out on a Sabouraud agar inclined plate, and periodically transferred to a Sabouraud agar plate, and cultured at 28°C for 48 hours. In order to prepare the quiescent yeast, the colonies were picked from the agar plate, transferred to 30 ml Sabouraud glucose liquid medium, and cultured at 32°C for 72 hours. The obtained cells were centrifuged at 1000xg for 10 minutes, and the deposited cells were washed twice with distilled water. Then count the number of cells and suspend them in Hank's Balanced Salt Solution (HBSS) to the required concentration. Through the 0.01% methyl blue efflux test to determine the viability of bacteria. The results showed th...
Embodiment III
[0050] The purpose of this example is to compare the antibacterial activity of α-MSH and / or its derivatives with fluconazole, which is an approved antifungal drug.
[0051] Detection of α-MSH (1-13) (serial number 4), (4-10) (serial number 2), (6-13) (serial number 3), (11-13) (serial number 1 ) And ACTH (1-39), (18-39) and fluconazole in 10 -6 -10 -4 The ability to resist Candida albicans at M concentration is the same as in Example II. Figure 4 shows that compared with fluconazole, α-MSH (11-13) (serial number 1), (4-10) (serial number 2), (6-13) (serial number 3) and (1-13) (Sequence No. 4) is very effective against Candida albicans, and its inhibitory activity is similar to that of fluconazole at equimolar concentrations. In contrast, the core α-MSH sequence (4-10) (sequence number 2) with behavioral effects but very little anti-inflammatory activity produced nearly 50% of the colony forming unit inhibitory effect. Although this inhibitory effect is significant (p-4 At M conce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com