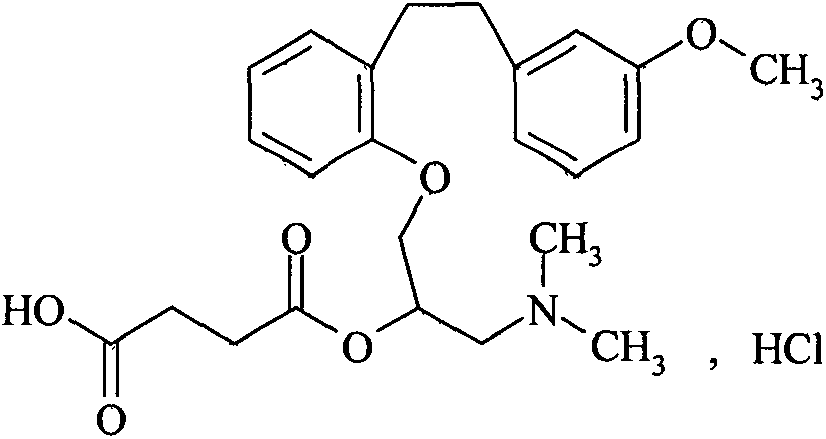
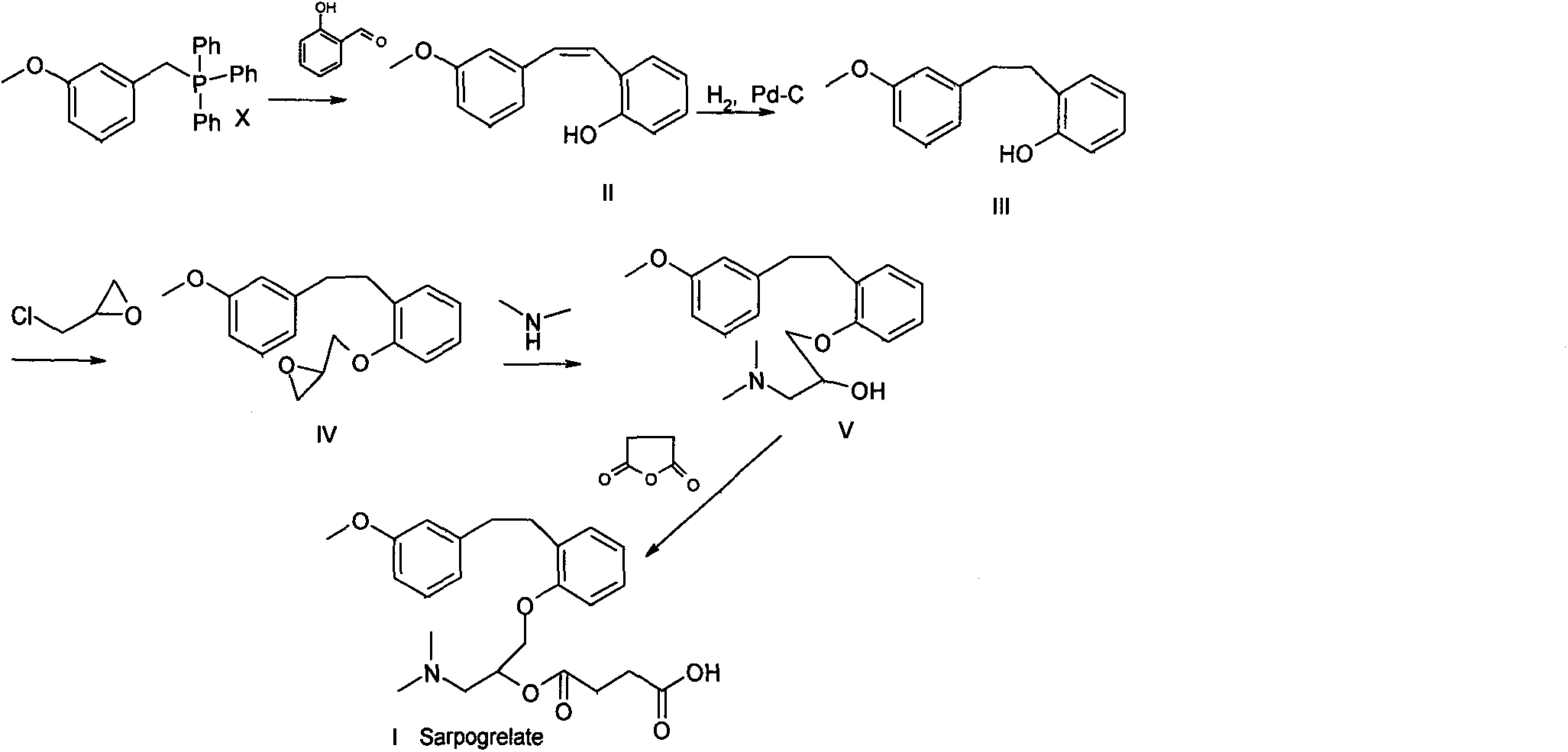
Method for preparing sarpogrelate hydrochloride
A technology of sarcogrelate hydrochloride and salt formation, which is applied in the field of preparation of sarcogrelate hydrochloride, can solve the problems that it is difficult to obtain high-purity products and difficult to remove, and achieve the effect of simple industrial production, easy operation and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] 100 kg (containing 55% of triphenylphosphine oxide) of 2-((3-methoxy) styryl) phenol mixture prepared by Wittig reaction (J.Med.Chem.1990, 33:1818-1823) Into a 1000L autoclave, add 450 liters of ethanol, 15 kg of 5% Pd-C, add hydrogen to 0.5Mpa, stir and hydrogenate at a temperature of 50°C for 3 hours, filter and concentrate to obtain a light yellow oil with a yield of 96%, HPLC Detection showed complete hydrogenation. Example 22-((3-methoxy)phenethyl)phenol (mixture)
Embodiment 2
[0039] Add 190 grams of 2-((3-methoxy) styryl) phenol mixture (containing 52% triphenylphosphine oxide) prepared by Wittig reaction into the reaction flask, add methanol 1000 ml, 20 grams of Raney nickel, Introduce hydrogen, stir and hydrogenate at 40°C for 10 hours, filter, and concentrate to obtain a light yellow oil, which is detected by HPLC and shows that the hydrogenation is complete. Example 32-((3-methoxy)phenethyl)phenol (mixture)
Embodiment 3
[0040] As in Example 1, 10% palladium carbon was used instead, the pressure was 1 MPa, and the reaction was carried out at room temperature, with a yield of 95%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com